PET Probe Lights Up MCI, AD
Quick Links
A new study in yesterday’s New England Journal of Medicine shows that PET imaging using the amyloid-binding agent FDDNP can distinguish between normal healthy subjects, and those with either AD or the pre-AD condition of mild cognitive impairment (MCI). In a small group of patients, FDDNP-PET scans also showed increased labeling after 2 years in those whose disease progressed.
The results, from Gary Small, Jorge Barrio, and colleagues from the David Geffen School of Medicine at the University of California at Los Angeles, along with the encouraging success with the amyloid imaging agent Pittsburg compound B (see ARF related news story), are a welcome advance in efforts to develop non-invasive methods to track the progression of AD pathology, and to eventually evaluate the efficacy of anti-amyloid therapies.
Barrio and colleagues have developed FDDNP, a derivative of the hydrophobic, solvent-sensitive, fluorescent probe 2-{1-[6-(dimethylamino)-2-naphthyl]ethylidene}malononitrile (DDNP). The compound labels amyloid plaques and, to a lesser extent, tangles, in brain slices in vitro (Agdeppa et al., 2001), and shows higher retention in brains of AD patients compared to normal controls (see ARF related news story). FDDNP labeling of protein deposits is closely related to the amyloid properties of these aggregates and labels amyloid-β plaques and tau paired helical filaments, which are present in AD, but not the tau straight filaments found in Pick’s and other neurologic diseases (see Smid et al., 2006). In this study, Small and colleagues used 18F-FDDNP to image 83 volunteers, who also underwent extensive neuropsychological testing and clinical evaluation leading to a diagnosis of cognitive normal (25 subjects), MCI (28), or AD (30). The researchers also measured 2-deoxyfluoroglucose uptake in subjects, and 72 underwent MRI.
The MCI group had elevated FDDNP retention compared to normal subjects, and the AD group was higher still. The differences between the groups were small but highly statistically significant. The data within groups showed a very tight distribution, which may be a strength of the method. But it could also be partly due to the careful selection of subjects—the 83 came from an initial pool of 737 volunteers with self-reported memory problems. One caveat to the study is that the control group was significantly younger than either the MCI or AD groups, with an average age of 64 compared to 70 for MCI and 73 for AD.
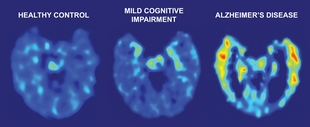
PET Tracer Reveals Alzheimer Pathology
Brain PET scans from a healthy volunteer (left), a subject with mild cognitive impairment (middle), and a subject with Alzheimer disease (right). Red and yellow areas show the retention of FDDNP, an amyloid imaging agent developed by Jorge Barrio and colleagues at the University of California at Los Angeles. A study of 83 subjects found a significant increase of FDDNP labeling in subjects with MCI over a two year period. Image credit: UCLA
Regional increases of FDDNP binding were detected in the groups with MCI and AD in every part of the brain examined, which included the temporal, parietal, posterior cingulate, and frontal regions. These results suggest the method may be useful to measure regional plaque load.
Other accepted measures of pathology, the glucose uptake measure and medial temporal volume, also showed changes between normal, MCI, and AD subjects. Of all the measures, the global FDDNP binding values best distinguished the three groups. Lower FDDNP binding correlated with higher scores on the Mini-Mental State Exam.
One subject with AD died 14 months after the PET scan, giving the investigators an opportunity to have a direct look at brain pathology. They found that regions with high FDDNP showed elevated plaques and tangles, supporting the relation between PET detection and pathological load.
Two years later, they reanalyzed 12 subjects—eight control and four with MCI—to see if the FDDNP might detect disease progression. One control subject who progressed to MCI did show increased FDDNP, as did another who showed a decline in test scores but did not reach the diagnostic criteria for MCI. Of four subjects with MCI, two who progressed to AD had increased FDDNP retention, while two that remained cognitively stable did not. While the numbers are small, the results are encouraging to researchers and clinicians looking for both early detection and a way to track the clinical effects of potential anti-amyloid treatments. In an e-mail, Small told Alzforum that other researchers are very interested in trying out FDDNP, and plans are underway to expand the technique to more sites.—Pat McCaffrey
References
News Citations
- Pittsburgh Compound-B Zooms into View
- New PET Probe to Aid Diagnosis and Monitoring of Alzheimer's Disease
Paper Citations
- Agdeppa ED, Kepe V, Liu J, Flores-Torres S, Satyamurthy N, Petric A, Cole GM, Small GW, Huang SC, Barrio JR. Binding characteristics of radiofluorinated 6-dialkylamino-2-naphthylethylidene derivatives as positron emission tomography imaging probes for beta-amyloid plaques in Alzheimer's disease. J Neurosci. 2001 Dec 15;21(24):RC189. PubMed.
- Smid LM, Vovko TD, Popovic M, Petric A, Kepe V, Barrio JR, Vidmar G, Bresjanac M. The 2,6-disubstituted naphthalene derivative FDDNP labeling reliably predicts Congo red birefringence of protein deposits in brain sections of selected human neurodegenerative diseases. Brain Pathol. 2006 Apr;16(2):124-30. PubMed.
Further Reading
Papers
- Kepe V, Huang SC, Small GW, Satyamurthy N, Barrio JR. Visualizing pathology deposits in the living brain of patients with Alzheimer's disease. Methods Enzymol. 2006;412:144-60. PubMed.
- Lockhart A. Imaging Alzheimer's disease pathology: one target, many ligands. Drug Discov Today. 2006 Dec;11(23-24):1093-9. PubMed.
Primary Papers
- Small GW, Kepe V, Ercoli LM, Siddarth P, Bookheimer SY, Miller KJ, Lavretsky H, Burggren AC, Cole GM, Vinters HV, Thompson PM, Huang SC, Satyamurthy N, Phelps ME, Barrio JR. PET of brain amyloid and tau in mild cognitive impairment. N Engl J Med. 2006 Dec 21;355(25):2652-63. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Austin Hospital
This paper shows that imaging of specific pathology with PET tracers provides diagnostic advantages over non-specific measures, such as atrophy on MRI and hypometabolism on FDG PET, that should lead to increased accuracy in the diagnosis of AD and much earlier diagnosis. However, there is room for substantial improvement in ligands for amyloid and tau imaging. FDDNP only showed a 9 percent increase in binding in AD compared to controls. Despite this small increase, the scan was able to distinguish all AD from controls due to very low variance in the groups and very low test-retest variation. This required a 2-hour scan that is not practical for widespread clinical application and it may be difficult for other groups to reproduce this level of precision. In contrast, C-11 PIB shows an 80-100 percent increase in cortical binding in AD compared to controls, and a simple delayed image of 20-30 minutes’ duration has been validated as an alternative to DVR. PIB images should be easier for clinicians to read. PIB is more specific, binding only to Aβ plaques, and therefore more appropriate for assessment of therapies designed to alter Aβ levels. The disadvantage of C-11 PIB is the short half-life of the radioactive tag that requires each dose to be produced from a cyclotron immediately before each scan. Several F-18 tracers with properties similar to C-11 PIB are entering human trials. My group has studied 170 subjects with C-11 PIB including 50 with MCI. The greater dynamic range of PIB has allowed clear distinction within the MCI subjects of a group with normal uptake (35 percent of MCI subjects) with the rest predominantly falling into the lower range of AD subjects. This may allow prediction of those that will progress to AD from those who will not. It is generally accepted that 30-40 percent of persons given a diagnosis of MCI will improve or remain stable and that they are unlikely to have underlying AD. Longitudinal studies are underway to confirm or refute this proposal.
In summary, the paper by Small et al. is welcome news for those seeking a test to improve the accuracy and early diagnosis of AD. Improvements in tracer design should allow this technology to become a useful and widely available clinical tool.
Karolinska Institutet
In this study, the authors have used a somewhat unusual approach to recruit patients to take part in PET studies of Alzheimer disease (AD) and mild cognitive impairment (MCI). They have made advertisements about the study, including media coverage. From an initial sample of 737 volunteers, they included 25 AD, 28 MCI, and 30 controls in the study. All subjects who volunteered for the study described subjective memory problems. The 30 controls selected among the volunteering subjects have been found to have no measurable cognitive impairment. These 30 subjects would by many clinicians be called “subjects with subjective memory problems (subjective MCI),” but not considered to be controls.
Since some of the MCI patients were on cholinesterase inhibitor treatment, it might be possible that due to treatment effect they are not correctly classified as AD based on the cognitive tests.
There is a follow-up of 12 controls and four MCI subjects (two converted to AD) both with PET and cognitive testing. Since only two out of 28 MCI subjects are reported to convert to AD during 24 months, the conversion rate is very low.
The differences between the PET ligand FDDNP and PIB have been widely discussed since the initial papers on FDDNP (Shoghi-Jadid et al., 2002; Klunk et al., 2004) and concerns have been raised about the sensitivity of FDDNP. This is also illustrated in the paper of Small et al. where the authors report significant differences between control/MCI/AD, but the differences between changes in control and AD/MCI 5-10 percent should be compared to the robust differences between controls/AD/MCI with PIB of 40-70 percent (Klunk et al., 2004; Engler et al., 2006; Nordberg et al., 2006 IDAC Madrid 2006).
The authors report a significant difference between FDDNP binding in MCI versus AD patients. As illustrated in Figure 2 there is a broad variation in FDDNP binding in the MCI group overlapping both the AD and control group as well (see medial temporal cortex). The authors do not discuss whether there might be two groups of MCI patients—those who show FDDNP binding comparable with MCI, and those MCI patients with FDDNP binding comparable with AD. This is a phenomenon discussed for PIB binding in MCI (Price et al., 2005; Nordberg et al., ICAD Madrid 2006). The histopathological studies performed in one autopsy case with earlier FDDNP PET investigation showed abundance of especially immunoreactive tangles in the medial temporal regions. These observations may suggest that FDDNP might be a more sensitive marker for tangles than for amyloid plaques. If this is the case, we presently may have two PET ligands, namely PIB (amyloid plaques) and FDDNP (tangles), that could be quite useful for both the understanding of the time course of disease progression as well as for evaluation of new treatment strategies. The multi-tracer PET studies, which could also include tracers for inflammation processes and neurotransmitter function, would then provide a deeper understanding of the pathophysiological disease processes that we now, 100 years after Alois Alzheimer, are starting to understand in living AD patients.
I do not think we should discuss choosing between these two PET ligands. They probably are a good complement to each other and further evaluation is needed, especially now in subjects with hereditary forms of AD as well as in those with other forms of dementia. The PET ligands should also now be applied to the different types of anti-amyloid therapy that are being evaluated presently.
References:
Shoghi-Jadid K, Small GW, Agdeppa ED, Kepe V, Ercoli LM, Siddarth P, Read S, Satyamurthy N, Petric A, Huang SC, Barrio JR. Localization of neurofibrillary tangles and beta-amyloid plaques in the brains of living patients with Alzheimer disease. Am J Geriatr Psychiatry. 2002 Jan-Feb;10(1):24-35. PubMed.
Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergström M, Savitcheva I, Huang GF, Estrada S, Ausén B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Långström B. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004 Mar;55(3):306-19. PubMed.
Engler H, Forsberg A, Almkvist O, Blomquist G, Larsson E, Savitcheva I, Wall A, Ringheim A, Långström B, Nordberg A. Two-year follow-up of amyloid deposition in patients with Alzheimer's disease. Brain. 2006 Nov;129(Pt 11):2856-66. PubMed.
Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, Holt DP, Meltzer CC, Dekosky ST, Mathis CA. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab. 2005 Nov;25(11):1528-47. PubMed.
University of Goteborg, Sahlgrenska University Hospital
FDDNP-PET shows promise for monitoring plaque and tangle pathology in Alzheimer patients
Research advances on the molecular pathogenesis of Alzheimer disease (AD) have led to several drug candidates with potential disease-modifying effects, for example, secretase inhibitors and β amyloid immunotherapy. If any of these drugs prove to have a clinical effect, they are likely to have the best efficacy in the early phase of the disease, when the neuronal degeneration has not become too widespread. Thus, there is a great need for diagnostic tools, often called biomarkers, which will enable early and accurate diagnosis of AD. Especially, biomarkers allowing the identification of incipient AD already in patients with mild cognitive impairment (MCI) would be of great value.
However, considering the large variation in the distribution and severity of both neuropathological changes and neurochemical abnormalities among AD cases, it is unlikely that any single biomarker will fulfill the requirements of high enough sensitivity and specificity. Instead, combinations of biomarkers, each reflecting different aspects of the neuropathological and neurochemical processes, will probably prove to be useful. Indeed, a number of promising diagnostic tools have to a different extent been validated for their capacity to identify AD early in the disease process, including the cerebrospinal fluid (CSF) biomarkers total tau (T-tau), phospho-tau (P-tau) and Aβ42; magnetic resonance imaging (MRI) measurements of hippocampal and entorhinal cortex atrophy; fluoro-deoxy-glucose (FDG) positron emission tomography (PET) measurements of regional abnormalities in glucose and oxygen metabolism; and visualization of β amyloid deposition using Pittsburgh Compound-B (PIB) PET [1].
In this paper, Gary Small and coworkers introduce a new PET method using the tracer 2-(1-{6-[(2-[F-18]fluoroethyl)(methyl)amino]-2-naphthyl}ethylidene)malononitrile (FDDNP), which binds to both plaques and tangles. In AD cases, FDDNP binds to cortical brain regions known to be affected by plaques and tangles.
In the study, data on FDDNP binding were presented as relative distribution volumes (DVR), which is the tracer distribution in the region of interest (ROI) divided by the distribution volume in the reference region (the cerebellum). In AD and MCI cases, approximately 10 percent higher DVRs were found in several cortical brain regions. Since the standard deviation (SD) within each diagnostic group was notably small, the estimated effect sizes (i.e., the difference between the group means divided by the pooled SD) were high, between 2.5 and 4.5.
The diagnostic accuracy of the method, evaluated by receiver operating characteristics (ROC) curves, was very high; 0.95 and 0.98 to differentiate MCI and AD from controls. Interestingly, ROC values for FDDNP were clearly higher than for either FDG-PET or MRI measurements of hippocampal volume.
Thus, the new FDDNP-PET shows great promise as a diagnostic tool for MCI and AD. FDDNP-PET may also be useful as a surrogate marker in clinical trials on the new type of drug candidates with disease-modifying potential, both in trials on drugs targeting Aβ deposition and tangle formation. Indeed, biomarker data from small clinical trials suggesting that a drug has positive effects on these pathogenic processes would be of great value to make a go/no-go decision for an expensive clinical trial with clinical improvement as the endpoint.
The research community will be waiting for future studies on the clinical usefulness of FDDNP-PET imaging. First of all, we need replication studies on prospective patients with lower dropout rates than in the present study, and also studies including cases with other dementia disorders such as frontotemporal dementia, Lewy body dementia, and Creutzfeldt-Jakob disease. It will also be interesting to see a direct comparison of the regional binding of the two ligands for plaques (PIB) and plaques and tangles (FDDNP). Further, since a recent study showed that all subjects, regardless of clinical status, with positive PIB binding had low CSF Aβ42 levels, and vice versa [2], it will also be interesting to learn how FDDNP binding correlates not only to CSF Aβ42 levels, but also to CSF T-tau and P-tau levels.
A last linguistic comment on the paper is that since the term “invasive” is defined as “puncture of the skin with entry of foreign material into the body as part of a diagnostic technique,” and FDDNP-PET is based on the injection of a radioactive substance into the body through an indwelling venous catheter, it may be considered unfortunate to claim FDDNP-PET as “a noninvasive method,” as in the conclusion of the Abstract. Nevertheless, FDDNP-PET is a promising new clinical tool for visualization of plaques and tangles directly in living patients, and is likely to provide useful diagnostic information in early AD.
References:
Blennow K, de Leon MJ, Zetterberg H. Alzheimer's disease. Lancet. 2006 Jul 29;368(9533):387-403. PubMed.
Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, Larossa GN, Spinner ML, Klunk WE, Mathis CA, Dekosky ST, Morris JC, Holtzman DM. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006 Mar;59(3):512-9. PubMed.
University Hospital, Montevideo, Uruguay Faculty of Sciences
I want to congratulate the authors for this interesting, complex, well-performed study. It is stimulating to see that great effort is undertaken in the field of the PET technology to detect in vivo, pathological changes occurring in neurodegenerative diseases.
In general, I agree with the comments by Professors Rowe, Blennow, and Nordberg.
In my opinion, the strongest potential of FDDNP is the capability to detect neurofibrillary tangles, whereas PIB remains as a strong amyloid marker with a high level of discrimination between subjects with amyloid deposition and subjects without it (90 percent difference in parietal cortex!). More study must be done to clarify the future role of these two novel tracers in clinical routine as well as in the follow-up of different treatments. Meanwhile, here are some reflections:
1. PET is not a precision tool. For a PET tracer in clinical routine, its level of discrimination is an important factor. The long scanning time and the small percent difference between groups represent to me disadvantages of FDDNP compared with PIB.
2. For differential diagnosis and for monitoring drug treatments, the specificity of binding of the tracer is also important. The new approaches for treatment of AD concentrate on anti-amyloid drugs or drugs blocking the production of hyperphosphorylated tau. Because of this, it is important to differentiate between amyloid and neurofibrillary tangles. It might be a disadvantage for FDDNP to bind to both substances. But FDDNP might be useful to detect general signs of neurodegeneration. However, the binding to tangles in people older than the controls who participated in the study must be studied.
3. In my opinion, the comparison between FDDNP and FDG should have been done using the regional cerebral metabolic rate of glucose (rCMR glc) instead of SUVr. Variations in plasma glucose produce important changes in uptake.
My conclusion is that the study by Small et al. confirms that we are at the beginning of a new imaging era, in which we may be able to understand for the first time complicated pathological processes in vivo that we are today just scratching on the surface.
View all comments by Henry EnglerThis comment was co-authored by Gary Small, Henry Huang, Vladir Kepe, and
Jorge Barrio
We appreciate the interest that our paper has generated and wish to comment on some of the previous observations and clarify some points. In our New England Journal of Medicine paper (2006;355;2652-2663), we used a full 120 minute scan time for the purpose of validation of the quantification method, but we have also evaluated successfully the reduction of the FDDNP scan time from 120 to 60 minutes. For routine applications, we have found that a late scan reading between 30 to 60 minutes is not only sufficient but makes the procedure very easy to tolerate.
As presented in the paper, we have performed a brain autopsy in one of the patients scanned with FDDNP. Whereas tangles were found predominantly in the medial temporal lobe, plaques were the predominant pathology in the rest of the brain, confirming data in the literature. With knowledge of the distribution of plaques and tangles in a degenerating brain, FDDNP-PET could be used as a surrogate marker to monitor either anti-plaque or anti-tangle treatments or both. For an anti-tangle treatment, investigators would predict a greater effect size in the medial temporal region; for an anti-plaque treatment, the effect size would be greater in other cortical areas. Thus, FDDNP offers potentially great versatility as a surrogate marker tool.
Our approach to recruitment was far from unusual but rather typical for obtaining a convenience sample for study. The fact that our older controls had mild memory complaints is not unusual; in fact, nearly all individuals note subjective slowness in retrieval and learning with age. As pointed out in the paper, their self-acknowledged age-related memory complaints could have led to higher FDDNP-PET binding values since concern about memory complaints could be a subtle indication of pre-symptomatic disease in some controls. Despite this potential bias, we found that FDDNP-PET binding values for the control group were significantly lower than those for the MCI and the AD groups. Thus, these controls were clearly a distinct group from the MCI subjects who had more advanced objective cognitive decline. It is possible that some subjects were misclassified because of cholinesterase inhibitor treatment; however, when we eliminated subjects taking memory enhancing drugs from the analysis, our results did not change.
The statement in one of the comments that only two of 28 subjects converted to MCI is incorrect since we did not have available follow up data on all 28 subjects. Of the four MCI subjects available for follow up, two converted AD, which is consistent with the expected conversion rate over the follow-up period (30 months) in this study for the MCI subjects.
We agree with Dr. Blennow that a combination of biomarkers may be the best approach. Although we are using a combination of molecular imaging probes to properly evaluate the many variables associated with AD (e.g., neuropathological aggregates, neuronal losses and dysfunction), the added cost of multiple markers will have to be weighed against the added value of each measure when the approaches become routine clinical tools.
The word “non-invasive” classically describes all PET imaging approaches. Brain biopsy or sampling of CSF in patients are more invasive approaches to document brain amyloid and tau in living patients than a PET scan.
We agree that studies of FDDNP and PIB in the same subjects will further help us to understand these novel imaging approaches in living subjects, and such studies are currently underway at several sites.
View all comments by Gary W. SmallMake a Comment
To make a comment you must login or register.