Phase 2 Data of AADvac1 in Alzheimer’s Disease Published
Quick Links
Results from a Phase 2 trial of AADvac1—a vaccine against pathological forms of tau—were published June 14 in Nature Aging. The treatment met its primary endpoint, appearing safe. It also rallied antibody responses among nearly all participants, who had been diagnosed with mild Alzheimer’s disease, and attenuated a gradual rise in plasma NfL over the course of the two-year-trial. Among a small subset of volunteers who had cerebrospinal fluid drawn, the vaccine reduced the concentration of CSF p-tau217, and trended toward lowering p-tau181 and total tau as well. Alas, the vaccine did not slow cognitive decline overall, although post hoc analysis suggested that it did so among those most likely to harbor Aβ and tau pathology.
- AADvac1 appeared safe and stoked an antibody response.
- The vaccine lessened increases in plasma NfL, and lowered CSF p-tau-217.
- Cognitive decline continued unabated.
- A larger Phase 2b trial is in the works.
Led by Petr Novak of Axon Neuroscience in Bratislava, Slovakia, the study comes on the heels of the U.S. FDA’s controversial approval of aducanumab. That approval rested on statistically fraught Phase 3 trial data that was never published—a top criticism by researchers in the field.
Alzforum covered the bulk of the AADvac1 findings when they were presented at the AD/PD meeting last year (Apr 2020 conference news). In short, the trial, called ADAMANT, enrolled 196 participants who had mild AD as per NIA-AA criteria, along with either medial temporal lobe atrophy by MRI, or CSF biomarker evidence of Aβ or tau pathology. Participants were randomized 3:2 to receive 11 doses of 40 mg AADvac1, or a placebo that contained adjuvant but no vaccine. They received the first six subcutaneous injections monthly, followed by five quarterly boosters. The trial started with 117 participants in the active group and 79 in the placebo group. Seventeen percent dropped out during the trial, leaving 100 in the active and 63 in the placebo group.
As reported at the conference, the trial met its primary objective of safety and tolerability. Only injection-site reactions, and confusion, were more common in the treatment group. While no one in the placebo group reported confusion, six people in the active group did. For five of them, this symptom was transient. Two people in the active group died, but their causes of death were deemed unrelated to the vaccine.
The paper includes more detailed data on the immunogenicity of AADvac1, a secondary outcome. After the first six doses, 96.5 percent of participants in the treatment group produced detectable levels of IgG antibodies, predominantly of the IgG1 isotype. They were specific for Axon Peptide 108—the tau epitope within AADvac1. At their peak, after six doses of vaccine, antibody concentrations reached an average of nearly 2 μg/mL in the plasma. Antibody concentrations dropped by nearly half during the intervals between booster vaccinations, which took place every 14 weeks following the sixth dose. The boosters restored titers to peak concentrations. Antibody levels in the CSF averaged about 0.3 percent of those in the serum. Notably, younger participants posted a more robust response to the vaccine than older volunteers.
Alas, the vaccine did not budge measures of cognition. Participants in treatment and placebo groups slipped by similar amounts on the clinical dementia rating scale sum of boxes (CDR-SB) and the Alzheimer’s Disease Cooperative Study (ADCS)-mild cognitive impairment (MCI)-Activities of Daily Living (ADL).
The trial included several exploratory biomarker measurements. Plasma NfL increased in both groups over the 104-week trial, but less so in the vaccine group. While the neurodegeneration marker rose by 27.7 percent in the placebo group, it inched up by 12.6 percent in the active group.
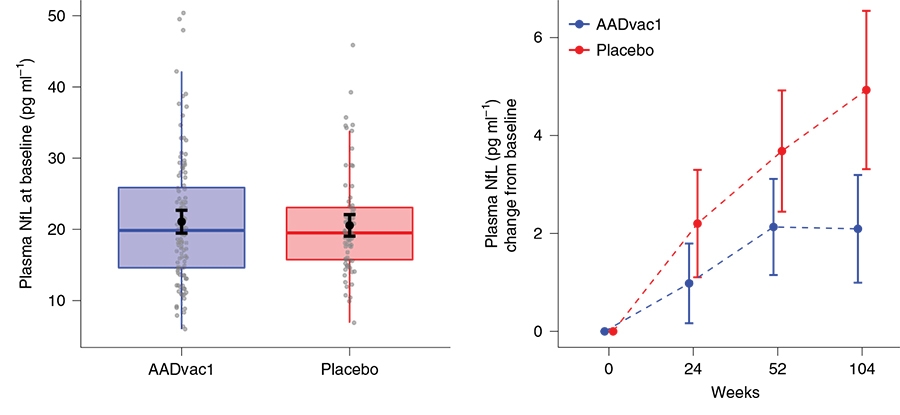
Slower Degeneration? Plasma NfL concentration rose from baseline (left) in both groups over the 104-week trial, but at a slower pace among people in the active group (right), stabilizing during the second year. [Courtesy of Novak et al., 2021.]
Only 27 participants—20 in the active and seven in the placebo group—had CSF biomarker measurements taken at baseline and at the end of the trial. Among them, the vaccine significantly reduced p-tau217, and trended toward lowering p-tau181 and total tau as well.
The vaccine did not influence measures of brain volume, whether measured across the whole brain or within specific regions. The researchers used diffusion tensor imaging to measure white-matter integrity in a subset of 20 participants, including 13 from the treatment and seven from the placebo groups. AADvac1 appeared to stabilize the fornix, a white-matter tract that originates from the hippocampus and is known to erode during AD. Among those in the placebo group, this tract degenerated over the course of the trial.
Of 46 participants who donated CSF at baseline, 15 did not meet all of the biomarker criteria for AD, which was based on having less than 600 pg/mL Aβ42, more than 400 pg/mL total tau, and greater than 60 pg/mL p-tau181. Thirteen of these participants met the Aβ42 and total tau cutoffs but not the p-tau-181 cutoff, while two patients did not meet Aβ42 or p-tau-181 cutoffs. These 15 patients were enrolled in the trial based on clinical diagnosis and atrophy in the medial temporal lobe, as were the 150 participants in the trial who did not have baseline CSF measurements taken. The findings suggested that a sizable proportion of participants in this trial might not meet the trial’s CSF biomarker criteria for AD.
Though it is not possible to know how many of the participants who did not donate CSF truly had AD, the researchers drew upon other available data from each participant to make an educated guess. A so-called multimodal classifier wove together demographic data, structural MRI, and clinical measures to identify a subset of participants most likely to harbor Aβ and tau pathology. In this subset of 109 participants, 93 completed the trial, including 54 in the active group and 39 in the placebo group. Among people in this AD-likely group, the vaccine slowed decline on the CDR-SB by 27 percent and on the ADCS-MCI-ADL by 30 percent. It also slowed the rise of plasma NfL, but did not influence brain atrophy.
Though plagued with the typical pitfalls of post hoc analyses, these findings hint that the vaccine could benefit people who are burdened by its target—pathological tau.
Axon plans to put AADvac1 to the test in a Phase 2b trial, which aims to enroll 400 participants with biomarker evidence of Aβ and tau pathology, Novak told Alzforum. “Given that aducanumab was approved by the FDA based on a surrogate biomarker outcome using the accelerated approval pathway, should this study be positive, we could explore possibilities for an accelerated approval,” he added. Axon is currently in discussion with potential partners for further development, and would like to start the trial by the end of the year.—Jessica Shugart
References
Therapeutics Citations
News Citations
Further Reading
No Available Further Reading
Primary Papers
- Novak P, Kovacech B, Katina S . ADAMANT: a placebo-controlled randomized phase 2 study of AADvac1, an active immunotherapy against pathological tau in Alzheimer’s disease. Nat Aging. 1, 2021, pp521-34. Nat Aging.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.