Prefrontal Hook-Up Required for New Memories in the Hippocampus
Quick Links
When it comes to forging lasting memories, the hippocampus relies on a little help from the prefrontal cortex. That is the conclusion from a study published July 28 in the Proceedings of the National Academy of Sciences. In it, researchers reported that beyond its known role of helping the hippocampus consolidate memories, the prefrontal cortex plays a key role in encoding the memories in the first place. Exposure to memorable experiences greatly activated neurons in the prefrontal cortex, and they started the process of memory-making via a tight circuit with the hippocampus. The findings may put the prefrontal cortex—a network hub that acquires Aβ plaques early in Alzheimer’s disease—squarely on the map of brain regions centrally involved in causing symptoms of memory loss in AD.
Scientists have long known that the hippocampus must integrate inputs from different regions of the brain to consolidate memories, but which parts of the brain provide those inputs, and how they drive memory formation, is still an open frontier in research. The prefrontal cortex (PFC) is a supremely connected network hub that links up with the hippocampus via a circuit that passes through the entorhinal cortex. Previous studies have implicated the PFC, particularly the medial PFC (mPFC), in the consolidation and storage of memories (see Maviel et al., 2004; Wiltgen et al., 2004; and Frankland and Bontempi, 2005). However, brain imaging indicated that the PFC may play a role in locking in memories from the get-go, as neurons in the PFC ramped up their activity when episodic memories were first formed (see Wagner et al., 1998).
“This prompted us to wonder what the function of this early prefrontal activation might be,” said first author Adam Bero of the Massachusetts Institute of Technology in Cambridge.
To tease out the contribution of the mPFC in memory encoding, Bero, senior author Li-Huei Tsai, and colleagues turned to fear conditioning in mice. In this classic memory model, researchers let mice explore a new environment in which they experience a brief foot shock. This creates an associative memory that manifests as a freezing behavior when the mice encounter that environment again. The researchers found that one hour following fear conditioning, neurons in the mPFC expressed elevated levels of zif268, a transcription factor that switches on following neuronal activation. It was not just zif268—RNA sequencing showed that 342 genes were differentially expressed in mice that underwent fear conditioning as compared to those that had merely explored a new cage. The upregulated genes tended to be involved in synaptic plasticity and memory formation, whereas many of the downregulated genes were known to be involved in processes that suppress those functions, such as microglial activation.
Bero and colleagues then tested whether these genetic activation markers translated into true synaptic remodeling in the mPFC. In the transmission electron microscope, the researchers saw that the synaptic active zones were enlarged, and more synaptic vesicles were docked in neuronal synapses in mPFC neurons following fear conditioning. The researchers also saw more thin dendritic spines—those thought to be involved in rewiring neuronal circuits after new experiences. Finally, single cell patch clamp experiments revealed a boost in postsynaptic excitatory currents in mPFC neurons shortly after the fear conditioning.
Did this early burst of neuronal activity in the mPFC play a role in encoding the memory? To find out, the researchers used optogenetics to selectively switch off neurons in the mPFC. Quieting mPFC neurons turned down neuronal activity in the hippocampus and the entorhinal cortex following fear conditioning, the researchers found, suggesting a functional link between these regions early in memory formation.
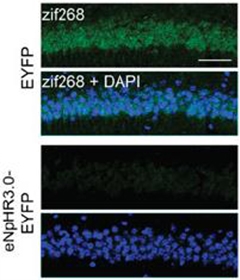
Drawing a Blank.
CA1 neurons in the hippocampus show a burst of activation (zif268 expression, green) during fear conditioning when the mPFC can fire signals. When neurons in the mPFC are shut down (bottom panels), the hippocampus stays quiet. [Image courtesy of Bero et al., PNAS.]
The grand finale experiment tested whether neurons in the mPFC were actually needed to form memories. The researchers optogenetically switched off mPFC neurons during fear conditioning, and then placed the mice back into the same environment later. Indeed, these mice showed less fear when placed back into the same environment one day or a month later, indicating that their fear-associated memories had not formed properly.
“This is an exciting study. It has long been recognized that memory consolidation is a hugely dynamic process,” wrote Paul Frankland of the Hospital for Sick Children in Toronto. “These authors show that changes in connectivity between neurons in the prefrontal cortex occur soon after learning and are necessary for successful long-term memory consolidation.”
Bero hypothesizes that the mPFC drives encoding of long-term memories by sending signals to the hippocampus. Following this initial coding, the hippocampus may then work with the mPFC to consolidate those memories, as has been shown in other studies.
Do the new findings have implications for the mechanisms behind AD-associated memory problems? Possibly, Bero said. “The prefrontal cortex is one of the first areas of the brain to develop Aβ plaques in Alzheimer’s disease, but we had no idea how those insults might contribute to memory impairment.” Bero thinks damage to the mPFC could lead to a loss of connectivity between the mPFC and hippocampus, which would derail the formation of new memories. Bero plans to test out this idea in future studies in AD model mice.—Jessica Shugart
References
Paper Citations
- Maviel T, Durkin TP, Menzaghi F, Bontempi B. Sites of neocortical reorganization critical for remote spatial memory. Science. 2004 Jul 2;305(5680):96-9. PubMed.
- Wiltgen BJ, Brown RA, Talton LE, Silva AJ. New circuits for old memories: the role of the neocortex in consolidation. Neuron. 2004 Sep 30;44(1):101-8. PubMed.
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005 Feb;6(2):119-30. PubMed.
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998 Aug 21;281(5380):1188-91. PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Bero AW, Meng J, Cho S, Shen AH, Canter RG, Ericsson M, Tsai LH. Early remodeling of the neocortex upon episodic memory encoding. Proc Natl Acad Sci U S A. 2014 Jul 28; PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.