Proteomics Point to RNA Splicing Defects in Alzheimer’s
Quick Links
Researchers believe they have discovered a new type of "tangle" in the Alzheimer’s brain: aggregates chock-full of RNA splicing machinery. Problems in RNA processing repeatedly surface in motor neuron disease. The new finding, reported in this week's Proceedings of the National Academy of Sciences online, makes the case that the same occurs in Alzheimer’s. Proteomics led scientists at Emory University in Atlanta, Georgia, to identify inclusions made up of spliceosome proteins in AD brains. These aggregates are accompanied by widespread defects in RNA splicing. While researchers knew that certain individual transcripts, such as tau, undergo altered splicing in AD, this study is the first to hint that the majority of RNAs may be at risk for mis-splicing. “Alterations in RNA processing might be a more global mechanism of neurodegenerative disease than previously thought,” said senior author Allan Levey.
The implications of this deficiency remain uncertain. Joint senior authors Levey, James Lah, and Junmin Peng, who has since left Emory for St. Jude Children’s Research Hospital in Memphis, Tennessee, collaborated on a large-scale project to analyze the insoluble proteome in seven different neurodegenerative conditions via mass spectrometry (see also Gozal et al., 2009). Joint first authors Bing Bai and Chadwick Hales of Emory, Yair Gozal at the University of Cincinnati, Ohio, and Ping-Chung Chen at St. Jude, describe one facet of that analysis, focusing on the protein set unique to AD. They compared cortical tissue from 10 people with late-stage Alzheimer’s with samples from 10 controls. They found 36 insoluble proteins that accumulated specifically in Alzheimer’s. These included the usual suspects—amyloid β (Aβ), Apolipoprotein E, and tau—as well as some newbies. For example, the researchers were intrigued to see several components of the U1 small nuclear ribonucleoprotein (U1 snRNP) aggregate as well. U1 contributes to the spliceosome complex that enacts the essential first steps of intron excision.
To characterize insoluble U1 snRNP in Alzheimer’s, the researchers located the complex in 30 AD brain samples with immunohistochemistry. In nearly all cases, the U1 snRNP appeared in “quite dramatic tangle-like structures,” said Lah. These cytoplasmic inclusions were absent from the brains of controls, as well as from brain samples of other tauopathies, making them a potentially specific Alzheimer’s marker, Lah said. Sometimes the U1 tangles overlapped with tau-containing aggregates, but not always (see image below).
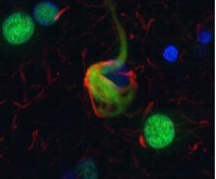
Splicing Machinery Tangles: In cortex from AD patient, U1-70K subunit (green) forms inclusions that partly co-localize with tau tangles (red). [Image courtesy of Bing Bai et al. © National Academy of Sciences]
Does U1’s entanglement impair RNA processing? A total loss of U1 function would likely be lethal, Lah reasoned, but a partial loss might affect the transcriptome. Specifically, the scientists looked for a higher-than-normal proportion of RNAs containing the first intron, which ought to be removed by the U1 complex. Normally these unspliced RNAs make up about one percent of overall mRNA, Lah estimated; RNA from AD brain contained about 1.5 percent. Overall, three-quarters of transcripts Lah looked at had an overabundance of unspliced forms in AD brains, compared to controls. “This appears to be a global effect,” Lah said.
As is often the case with findings from postmortem brain, the researchers do not know whether these inclusions are a cause or effect of AD. U1 could be considered a cause if researchers discovered people who have Alzheimer’s due to mutations in U1 complex members, Lah and Levey noted. No such mutations have yet been linked to familial AD. However, Lah noted that the small nuclear ribonucleoprotein polypeptide N , a core component of U1 and related complexes, was weakly if not significantly associated with AD in one genome-wide association study (see Naj et al., 2011). Lah suspects the U1 entanglement occurs downstream of Aβ pathology, and early during tau aggregation. The downstream effects of U1 pathology could be broad, since so many genes need it for splicing. U1 seems to be aggregated in the majority of AD cases, so a treatment to solve its problems could potentially be quite useful, Levey added. In addition, he suggested, the U1 spliceosome might serve as a biomarker in cerebrospinal fluid or on brain images.
Benjamin Wolozin of Boston University, who was not involved in the study, agreed that U1 could offer a new molecular marker. He suggested that U1 could cause disease in a similar manner to TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. These two proteins move from their normal location in the nucleus to the cytoplasm and miss the pre-mRNAs awaiting their attention in the nucleus. Wolozin and others have linked AD to RNA binding proteins, such as those found in stress granules (see ARF related news). It is unclear if the latter are related to U1 aggregates, but the new study makes a particularly robust association between AD and splicing, said Wolozin.—Amber Dance
References
News Citations
Paper Citations
- Gozal YM, Duong DM, Gearing M, Cheng D, Hanfelt JJ, Funderburk C, Peng J, Lah JJ, Levey AI. Proteomics analysis reveals novel components in the detergent-insoluble subproteome in Alzheimer's disease. J Proteome Res. 2009 Nov;8(11):5069-79. PubMed.
- Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, Buros J, Gallins PJ, Buxbaum JD, Jarvik GP, Crane PK, Larson EB, Bird TD, Boeve BF, Graff-Radford NR, De Jager PL, Evans D, Schneider JA, Carrasquillo MM, Ertekin-Taner N, Younkin SG, Cruchaga C, Kauwe JS, Nowotny P, Kramer P, Hardy J, Huentelman MJ, Myers AJ, Barmada MM, Demirci FY, Baldwin CT, Green RC, Rogaeva E, St George-Hyslop P, Arnold SE, Barber R, Beach T, Bigio EH, Bowen JD, Boxer A, Burke JR, Cairns NJ, Carlson CS, Carney RM, Carroll SL, Chui HC, Clark DG, Corneveaux J, Cotman CW, Cummings JL, DeCarli C, DeKosky ST, Diaz-Arrastia R, Dick M, Dickson DW, Ellis WG, Faber KM, Fallon KB, Farlow MR, Ferris S, Frosch MP, Galasko DR, Ganguli M, Gearing M, Geschwind DH, Ghetti B, Gilbert JR, Gilman S, Giordani B, Glass JD, Growdon JH, Hamilton RL, Harrell LE, Head E, Honig LS, Hulette CM, Hyman BT, Jicha GA, Jin LW, Johnson N, Karlawish J, Karydas A, Kaye JA, Kim R, Koo EH, Kowall NW, Lah JJ, Levey AI, Lieberman AP, Lopez OL, Mack WJ, Marson DC, Martiniuk F, Mash DC, Masliah E, McCormick WC, McCurry SM, McDavid AN, McKee AC, Mesulam M, Miller BL, Miller CA, Miller JW, Parisi JE, Perl DP, Peskind E, Petersen RC, Poon WW, Quinn JF, Rajbhandary RA, Raskind M, Reisberg B, Ringman JM, Roberson ED, Rosenberg RN, Sano M, Schneider LS, Seeley W, Shelanski ML, Slifer MA, Smith CD, Sonnen JA, Spina S, Stern RA, Tanzi RE, Trojanowski JQ, Troncoso JC, Van Deerlin VM, Vinters HV, Vonsattel JP, Weintraub S, Welsh-Bohmer KA, Williamson J, Woltjer RL, Cantwell LB, Dombroski BA, Beekly D, Lunetta KL, Martin ER, Kamboh MI, Saykin AJ, Reiman EM, Bennett DA, Morris JC, Montine TJ, Goate AM, Blacker D, Tsuang DW, Hakonarson H, Kukull WA, Foroud TM, Haines JL, Mayeux R, Pericak-Vance MA, Farrer LA, Schellenberg GD. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer's disease. Nat Genet. 2011 May;43(5):436-41. Epub 2011 Apr 3 PubMed.
External Citations
Further Reading
Papers
- Wolozin B. Regulated protein aggregation: stress granules and neurodegeneration. Mol Neurodegener. 2012;7:56. PubMed.
- Donovan LE, Higginbotham L, Dammer EB, Gearing M, Rees HD, Xia Q, Duong DM, Seyfried NT, Lah JJ, Levey AI. Analysis of a membrane-enriched proteome from postmortem human brain tissue in Alzheimer's disease. Proteomics Clin Appl. 2012 Apr;6(3-4):201-11. PubMed.
- Gozal YM, Dammer EB, Duong DM, Cheng D, Gearing M, Rees HD, Peng J, Lah JJ, Levey AI. Proteomic analysis of hippocampal dentate granule cells in frontotemporal lobar degeneration: application of laser capture technology. Front Neurol. 2011;2:24. PubMed.
- Mills JD, Janitz M. Alternative splicing of mRNA in the molecular pathology of neurodegenerative diseases. Neurobiol Aging. 2011 Nov 25; PubMed.
- Rudrabhatla P, Jaffe H, Pant HC. Direct evidence of phosphorylated neuronal intermediate filament proteins in neurofibrillary tangles (NFTs): phosphoproteomics of Alzheimer's NFTs. FASEB J. 2011 Nov;25(11):3896-905. PubMed.
- Zhou JY, Hanfelt J, Peng J. Clinical proteomics in neurodegenerative diseases. Proteomics Clin Appl. 2007 Nov;1(11):1342-50. PubMed.
- Xia Q, Cheng D, Duong DM, Gearing M, Lah JJ, Levey AI, Peng J. Phosphoproteomic analysis of human brain by calcium phosphate precipitation and mass spectrometry. J Proteome Res. 2008 Jul;7(7):2845-51. PubMed.
- Xia Q, Liao L, Cheng D, Duong DM, Gearing M, Lah JJ, Levey AI, Peng J. Proteomic identification of novel proteins associated with Lewy bodies. Front Biosci. 2008;13:3850-6. PubMed.
- Sultana R, Boyd-Kimball D, Cai J, Pierce WM, Klein JB, Merchant M, Butterfield DA. Proteomics analysis of the Alzheimer's disease hippocampal proteome. J Alzheimers Dis. 2007 May;11(2):153-64. PubMed.
- Liao L, Cheng D, Wang J, Duong DM, Losik TG, Gearing M, Rees HD, Lah JJ, Levey AI, Peng J. Proteomic characterization of postmortem amyloid plaques isolated by laser capture microdissection. J Biol Chem. 2004 Aug 27;279(35):37061-8. PubMed.
News
- Boosting Protein Translation PERKs Up Synapses in Alzheimer's Mice
- Paper Alert: Zebrafish Say TDP-43 Causes ALS by Loss of Function
- Yeast Models Say TDP-43 and FUS Are Not Cut From the Same Cloth
- All Aboard? SMA Protein Predicted to Organize Axonal RNA Transport
- Research Brief: Researchers Solicit SMN Understudy to Treat SMA
Primary Papers
- Bai B, Hales CM, Chen PC, Gozal Y, Dammer EB, Fritz JJ, Wang X, Xia Q, Duong DM, Street C, Cantero G, Cheng D, Jones DR, Wu Z, Li Y, Diner I, Heilman CJ, Rees HD, Wu H, Lin L, Szulwach KE, Gearing M, Mufson EJ, Bennett DA, Montine TJ, Seyfried NT, Wingo TS, Sun YE, Jin P, Hanfelt J, Willcock DM, Levey A, Lah JJ, Peng J. U1 small nuclear ribonucleoprotein complex and RNA splicing alterations in Alzheimer's disease. Proc Natl Acad Sci U S A. 2013 Oct 8;110(41):16562-7. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
The University of Queensland
This is interesting also in the context of our finding that in the presence of tau the splicing factor SFPQ is relocalized from the nucleus to the cytoplasm.
References:
Ke Y, Dramiga J, Schütz U, Kril JJ, Ittner LM, Schröder H, Götz J. Tau-mediated nuclear depletion and cytoplasmic accumulation of SFPQ in Alzheimer's and Pick's disease. PLoS One. 2012;7(4):e35678. PubMed.
Medical University of South Carolina
This is a truly exciting advance. While the small RNA world has captured the attention of the field, the global spicing failure is very novel and has been ignored since the days of KPI containing APP forms. It is curious that the authors saw a large increase in most (60 percent) of the MCI subjects tested, although only a relatively small fraction of them will end up getting AD. Does this mean that the deficit extends to other causes of MCI or that U1 protein dyshomeostais is an early and reversible defect?
Rutgers - New Jersey Medical School
This excellent paper brings back bittersweet memories from almost ten years ago, when we were captivated by the idea that RNA splicing in neurons could be altered in Alzheimer's by the availability of a C-terminal fragment of APP in the nucleus. In 2004, we proposed this notion in a paper that concluded that APP-specifically, a threonine-phosphorylated, C-terminal fragment (likely, AICD)-could regulate pre-mRNA splicing by localizing to the nuclear splicing factor compartment (SFC) [1].
The SFC is enriched in splicing factors (mostly SR proteins), snRNAs, and ribosomal proteins among others. It serves as storage and assembly site for the splicing machinery, regulating concentration and availability of splicing factors, and thus regulating pre-mRNA splicing [2, 3]. The SFC also contains transcription factors, and was proposed to play a role in regulating transcription [3].
The general belief at that time was that the C-terminal fragments of APP, in conjunction with the APP-binding protein Fe65, could regulate the transcription of AD-related genes [4-6]; less focus was given to their role in RNA splicing. Yet, the possibility that APP-derived polypeptides could participate in pre-mRNA splicing was–and still is-attractive to us. Our hypothesis on the dysregulation of pre-mRNA splicing in AD differs from that of Bai et al. Nevertheless, the idea that imbalanced APP metabolism leads to an excess of phosphorylated C-terminal fragment, and thus alters pre-mRNA splicing-as we thought-is compatible with the notion that AD-related conditions disrupt neuronal RNA processing. This in turn could play a role in AD pathogenesis, as Bai et al. now propose. Both lines of study are interesting, and need to be pursued.
References:
Muresan Z, Muresan V. A phosphorylated, carboxy-terminal fragment of beta-amyloid precursor protein localizes to the splicing factor compartment. Hum Mol Genet. 2004 Mar 1;13(5):475-88. PubMed.
Matera AG. Nuclear bodies: multifaceted subdomains of the interchromatin space. Trends Cell Biol. 1999 Aug;9(8):302-9. PubMed.
Misteli T. Cell biology of transcription and pre-mRNA splicing: nuclear architecture meets nuclear function. J Cell Sci. 2000 Jun;113 ( Pt 11):1841-9. PubMed.
Baek SH, Ohgi KA, Rose DW, Koo EH, Glass CK, Rosenfeld MG. Exchange of N-CoR corepressor and Tip60 coactivator complexes links gene expression by NF-kappaB and beta-amyloid precursor protein. Cell. 2002 Jul 12;110(1):55-67. PubMed.
Cao X, Südhof TC. A transcriptionally [correction of transcriptively] active complex of APP with Fe65 and histone acetyltransferase Tip60. Science. 2001 Jul 6;293(5527):115-20. PubMed.
Gao Y, Pimplikar SW. The gamma -secretase-cleaved C-terminal fragment of amyloid precursor protein mediates signaling to the nucleus. Proc Natl Acad Sci U S A. 2001 Dec 18;98(26):14979-84. PubMed.
Make a Comment
To make a comment you must login or register.