RNA-Binding Protein Controls Ataxin-1 Expression
Quick Links
Quantity matters: Too much or too little of certain proteins can result in neurodegeneration. A thorough understanding of how expression levels are regulated, from DNA to protein, could yield important clues about disease mechanisms, said Huda Zoghbi of Baylor College of Medicine in Houston. She and first author Vincenzo Gennarino illustrate this principle in a paper in the March 12 Cell, in which they describe a regulatory system that could be involved in spinocerebellar ataxia (SCA). A protein called Pumilio1 tightly regulates translation of the disease-linked ataxin-1 gene by destabilizing its RNA, they report. Zoghbi has not yet confirmed Pumilio1’s involvement in any human disease, but she suspects loss of the RNA-binding protein might underlie some childhood neurological disease.
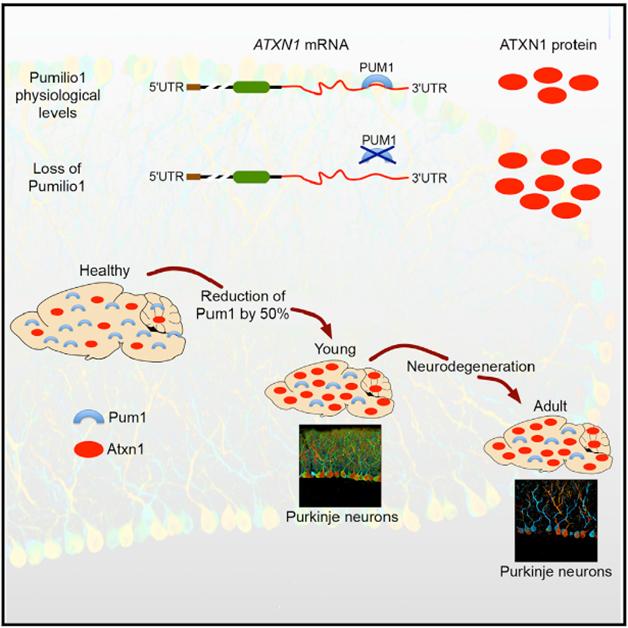
Pumilio1 Action. Loss of Pumilio1 results in too much ataxin-1 and cerebellar degeneration. [Image courtesy of Cell, Gennarino et al.]
“This spotlights the importance of going back to basics to understand everything we can about proteins involved in neurodegenerative diseases, including APP, α-synuclein, tau … to determine what regulates them transcriptionally, translationally, and post-translationally,” said Zoghbi. While researchers knew that RNA-binding proteins could regulate gene expression, Gennarino’s paper brings the issue to the fore, agreed Benjamin Wolozin of Boston University, who was not part of the study.
Ataxin-1 in SCA: Too Much of a Good Thing
Zoghbi’s laboratory has been studying the regulation of ataxin-1 in SCA for more than a decade. Ataxin-1 is a transcriptional repressor that influences expression of many other genes. Excess repeats of the trinucleotide CAG, encoding glutamine, in the ataxin-1 gene cause SCA type 1. Symptoms of cerebellar degeneration, including uncoordinated movements and loss of balance, appear in childhood or adulthood. Zoghbi and colleagues figured out that the repeats stabilize the ataxin-1 protein (Chen et al., 2003). It likely continues to do its regular job, Zoghbi said, but works overtime. Even a bit of extra ataxin1 causes disease, and lowering ataxin-1 by just 20 percent in SCA1 model mice carrying the expansion alleviates symptoms.
Gennarino noticed that the ataxin-1 mRNA possesses an unusually long 3’UTR—seven kilobases, folded into a complex configuration—and wondered if this twisted tail might be a site of regulation. Scanning the sequence with computer prediction programs, he discovered a motif for Pumilio1 binding. Not much is known about Pumilio1, Gennarino told Alzforum, beyond its role working with microRNAs to regulate genes involved in spermatogenesis and stem cell maintenance (Huszar, 2012; Joly et al., 2013). He confirmed that Pumilio1 bound to ataxin-1 mRNA, diminishing it in cultured human embryonic kidney cells and in mice.
Normally, Pumilio1 unwinds complex mRNAs to give access to regulatory microRNAs. In this case, however, Pumilio1 downregulated ataxin-1 even when Gennarino knocked down the microRNA silencing machinery, indicating Pumilio1 acted independently of microRNAs. Gennarino believes Pumilio1 binding promotes degradation of ataxin-1 mRNA, though he has not yet worked out the mechanism.
To check its relevance in vivo, Gennarino examined Pumilio1 knockout mice (Chen et al., 2012). Animals missing just one copy of the gene were uncoordinated, struggling to balance on a rotating rod at 5 weeks old. When he examined the brains from 10-week-old heterozygotes, he saw loss of the Purkinje cells in the cerebellum (see image above). In mice completely lacking Pumilio1, the movement disorder and neurodegeneration were more severe. Their phenotype was quite similar to that of SCA1 model mice with the ataxin-1 expansion, Gennarino said. Knocking down ataxin-1 by half in the Pumilio1-haplodeficient mice alleviated their deficits. The authors concluded that loss of Pumilio1 led to excess ataxin-1 and neurodegeneration.
If Pumilio1 also regulates ataxin-1 stability in people, then someone with Pumilio1 deficiency, or ataxin1 overexpression, would be susceptible to neurodegeneration, probably severe enough to begin in childhood, said Zoghbi. However, she has not yet found such an example. The work also suggests possible therapeutics for SCA, said Sheng-Han Kuo of Columbia University Medical Center in New York, who was not involved in the project. For example, he speculated, antisense oligonucleotides for ataxin-1 might alleviate disease by preventing translation. At least in mice, having lower-than-normal levels of ataxin-1 does not cause a big problem, Zoghbi said, because a protein called ataxin-1-like can take over most of its functions.
Kicking Off a Regulator 'Gold Rush'
The study’s implications go well beyond ataxin-1. Researchers studying neurodegeneration have focused on mutant versions of the aggregating proteins, and have only recently begun to appreciate that wild-type versions, when overabundant, accelerate disease, commented Aaron Gitler of Stanford University in Palo Alto, California, who was not involved in the paper. For example, duplications of the APP gene result in early onset Alzheimer’s (see Dec 2005 news), and duplications or triplications of α-synuclein cause Parkinson’s (see Nov 2013 news; Chartier-Harlin et al., 2004; Ibáñez et al., 2004). No one has examined the 3’UTR of these genes the way Gennarino did with ataxin-1, Zoghbi said, and that kind of study might yield new candidate disease genes. She also pointed out that genome-wide association studies often identify variants outside the coding region of genes. These variants might affect expression of disease-linked proteins, she said.
“I think this [paper] is going to spur a whole new gold rush in looking for regulators of neurodegenerative disease proteins like tau, APP, α-synuclein, and ataxin-2,” Gitler said. He has already begun to study the regulation of ataxin-2. As with ataxin-1, lengthy polyglutamine expansions in ataxin-2 cause SCA, but intermediate-sized expansions cause amyotrophic lateral sclerosis (see Aug 2010 news). The repeats also raise the levels of ataxin-2, but they confer stability on the protein itself, not its mRNA (Elden et al., 2010). Ataxin-1 intermediate-length expansions have also been linked to ALS, though not as conclusively as ataxin-2 has (Conforti et al., 2012; Spataro and La Bella, 2014).—Amber Dance
References
News Citations
- APP Double Dose Causes Early Onset AD
- Synuclein and Parkinson's—It's All in the Dose
- ALS—A Polyglutamine Disease? Mid-length Repeats Boost Risk
Paper Citations
- Chen HK, Fernandez-Funez P, Acevedo SF, Lam YC, Kaytor MD, Fernandez MH, Aitken A, Skoulakis EM, Orr HT, Botas J, Zoghbi HY. Interaction of Akt-phosphorylated ataxin-1 with 14-3-3 mediates neurodegeneration in spinocerebellar ataxia type 1. Cell. 2003 May 16;113(4):457-68. PubMed.
- Huszar G. Pumilio 1 control of spermatogenesis: a roadmap for future research. Asian J Androl. 2012 Sep;14(5):669. Epub 2012 Jun 18 PubMed.
- Joly W, Chartier A, Rojas-Rios P, Busseau I, Simonelig M. The CCR4 deadenylase acts with Nanos and Pumilio in the fine-tuning of Mei-P26 expression to promote germline stem cell self-renewal. Stem Cell Reports. 2013;1(5):411-24. Epub 2013 Nov 7 PubMed.
- Chen D, Zheng W, Lin A, Uyhazi K, Zhao H, Lin H. Pumilio 1 suppresses multiple activators of p53 to safeguard spermatogenesis. Curr Biol. 2012 Mar 6;22(5):420-5. Epub 2012 Feb 16 PubMed.
- Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destée A. Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet. 2004 Sep 25-Oct 1;364(9440):1167-9. PubMed.
- Ibáñez P, Bonnet AM, Débarges B, Lohmann E, Tison F, Pollak P, Agid Y, Dürr A, Brice A. Causal relation between alpha-synuclein gene duplication and familial Parkinson's disease. Lancet. 2004 Sep 25-Oct 1;364(9440):1169-71. PubMed.
- Elden AC, Kim HJ, Hart MP, Chen-Plotkin AS, Johnson BS, Fang X, Armakola M, Geser F, Greene R, Lu MM, Padmanabhan A, Clay-Falcone D, McCluskey L, Elman L, Juhr D, Gruber PJ, Rüb U, Auburger G, Trojanowski JQ, Lee VM, Van Deerlin VM, Bonini NM, Gitler AD. Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature. 2010 Aug 26;466(7310):1069-75. PubMed.
- Conforti FL, Spataro R, Sproviero W, Mazzei R, Cavalcanti F, Condino F, Simone IL, Logroscino G, Patitucci A, Magariello A, Muglia M, Rodolico C, Valentino P, Bono F, Colletti T, Monsurrò MR, Gambardella A, La Bella V. Ataxin-1 and ataxin-2 intermediate-length PolyQ expansions in amyotrophic lateral sclerosis. Neurology. 2012 Dec 11;79(24):2315-20. PubMed.
- Spataro R, La Bella V. A case of amyotrophic lateral sclerosis with intermediate ATXN-1 CAG repeat expansion in a large family with spinocerebellar ataxia type 1. J Neurol. 2014 Jul;261(7):1442-3. Epub 2014 Jun 11 PubMed.
Further Reading
Papers
- Delabar JM, Goldgaber D, Lamour Y, Nicole A, Huret JL, de Grouchy J, Brown P, Gajdusek DC, Sinet PM. Beta amyloid gene duplication in Alzheimer's disease and karyotypically normal Down syndrome. Science. 1987 Mar 13;235(4794):1390-2. PubMed.
- Crespo-Barreto J, Fryer JD, Shaw CA, Orr HT, Zoghbi HY. Partial loss of ataxin-1 function contributes to transcriptional dysregulation in spinocerebellar ataxia type 1 pathogenesis. PLoS Genet. 2010 Jul;6(7):e1001021. PubMed.
- Lee Y, Samaco RC, Gatchel JR, Thaller C, Orr HT, Zoghbi HY. miR-19, miR-101 and miR-130 co-regulate ATXN1 levels to potentially modulate SCA1 pathogenesis. Nat Neurosci. 2008 Oct;11(10):1137-9. PubMed.
- Ju H, Kokubu H, Lim J. Beyond the Glutamine Expansion: Influence of Posttranslational Modifications of Ataxin-1 in the Pathogenesis of Spinocerebellar Ataxia Type 1. Mol Neurobiol. 2014 Apr 22; PubMed.
News
- Toxicity of Polyglutamine Expansion Follows Normal Channels
- More Evidence for Dynamic PolyQ Aggregates
- Do Copy Number Variations Point to Potential AD Genes?
- Ps and Qs—Polyglutamines Not Necessary for Toxicity?
- Research Brief: Gain or Loss of Function? Ataxin Mutation Cuts Both Ways
- Neurodegeneration—A Developmental Problem?
Primary Papers
- Gennarino VA, Singh RK, White JJ, De Maio A, Han K, Kim JY, Jafar-Nejad P, di Ronza A, Kang H, Sayegh LS, Cooper TA, Orr HT, Sillitoe RV, Zoghbi HY. Pumilio1 haploinsufficiency leads to SCA1-like neurodegeneration by increasing wild-type Ataxin1 levels. Cell. 2015 Mar 12;160(6):1087-98. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.