Stop, Hey, What’s That Sound? ... Amyloid Is Going Down?
Quick Links
What it is ain’t exactly clear, but something's happening in the brain when sound waves rattle blood vessels—and it could potentially help treat Alzheimer’s, according to findings published in the March 11 Science Translational Medicine. Jürgen Götz at the University of Queensland in Brisbane, Australia, reported that ultrasound temporarily unsealed the blood-brain barrier (BBB), promoted amyloid plaque clearance, and rescued memory problems in a mouse model of AD. While the BBB has turned countless hopes into pipe dreams, as promising treatments buckled against its trusty endothelial walls, this study hints that simply opening the gates provides a therapeutic benefit unto itself. Götz and first author Gerhard Leinenga reported that opening the barrier somehow switched on microglia, which proceeded to gorge themselves with Aβ. Researchers in the field expressed cautious optimism that this method could safely breach the BBB, either to deliver drugs to treat AD or, as in this case, to motivate microglia.
“These findings probably fall under the rubric, a little bit of a bad thing can be good for you, but a lot of a bad thing is bad for you,” commented Terrence Town of the University of Southern California in Los Angeles. “They’ve tuned the ultrasound to the point where they can open the BBB and tickle microglia without doing damage, and under these conditions, it seems to be somewhat beneficial.” Town, who was not involved in the study, added that researchers will need to proceed with caution when applying this technique to the brains of aging people, whose BBB may already be fragile or somewhat compromised. Recent reports that the hippocampal BBB is progressively compromised with age and cognitive decline underscores that concern, he added (see Alzforum Webinar).

Ultrasonic Access.
Evans Blue dye seeped into the brain after researchers treated mice with ultrasound at a single spot (left), or scanned the entire brain with ultrasound (right). Infrared intensity ranges from blue to red. [Image courtesy of Leinenga and Götz, Science Translational Medicine, 2015.]
Researchers first used sound waves to bust through the BBB in rodents two decades ago, and later found they could open the barrier transiently and without cellular damage by delivering lower-energy sound waves along with microbubbles (see Vykhodtseva et al., 1995; Hynynen et al., 2001). Inert chambers of gas measuring 1-10 microns in diameter, microbubbles expand and contract to the rhythm of sound waves. When injected intravenously, they line the walls of blood vessels and capillaries, including those that make up the BBB. When ultrasound is delivered, the heaving oscillations of the bubbles ease tight junctions between endothelial cells and open the BBB (see Konofagou, 2012). In 2008, researchers used the technique to temporarily unlock the BBB in the hippocampus of AD mice and have since used it to deliver biomolecules and gene therapy vectors (see Choi et al., 2008; Nov 2009 conference story; Samiotaki et al., 2015; and Wang et al., 2015).
A recent study led by Kullervo Hynynen at the University of Toronto found that opening the barrier could trigger a therapeutic response all by itself. In the TgCRND8 AD mouse model, the researchers reported that targeting the hippocampus with ultrasound reduced plaque burden, improved spatial memory, and boosted the production of newborn neurons—all drug-free (see Burgess et al., 2014).
Götz and Leinenga sought to dive deeper into this ultrasonic phenomenon and get to the bottom of its mechanism. They first delivered a single ultrasound pulse to one region of the brain in normal mice that had been injected with microbubbles. Then they tested the barrier's integrity with Evans Blue, a dye that binds tightly to serum albumin, which normally cannot cross the BBB. Shortly after the ultrasonic pulse, the targeted 1mm area of the brain stained blue, indicating the barrier had been breached. Next, the researchers moved the ultrasound beam in 1.5mm increments across the entire brain (scanning ultrasound), and observed the dye throughout the brain 30 minutes later. Importantly, this widespread BBB opening did not allow red blood cells to enter the brain, and the researchers did not find evidence of cell death or ischemic damage the day after the treatment. Leinenga told Alzforum that the barrier had fully closed after two days.
The researchers next performed the same scanning ultrasound procedure on 10 APP23 transgenic mice at one year of age, when Aβ plaques are firmly established in the hippocampus. Another group of 10 mice received a sham treatment, in which they were injected with microbubbles and laid under the ultrasound machine without receiving the waves. The mice received four weekly treatments, and then Leinenga tested their spatial working memory using a Y maze. The ultrasound-treated mice performed as well as wild-type mice, whereas the sham-treated mice were slightly impaired. The researchers treated and then challenged a second group of mice with an active place-avoidance test, in which normal animals quickly learn to avoid a zone where they get a foot shock. Here too, the ultrasound-treated APP23 mice performed better than the sham-treated mice did, indicating that the treatment improved hippocampal-dependent spatial learning.
To help determine why the treatment improved cognition, Leinenga performed histological and biochemical analyses on the animals’ brains. Silver staining showed that the plaque burden was less than of half that in the cortex of sham-treated mice. Immunohistochemistry also indicated less plaque burden. Two Aβ-specific antibodies—4G8 and 6E10—revealed a marked reduction in several Aβ species, including total Aβ42, Aβ*56 oligomer, and high molecular weight species. In the second group of mice, the researchers also measured the amount of Aβ42 in the insoluble fraction, and found it was reduced by half in the ultrasound-treated mice.
Ralph Nixon of New York University commented that while the results certainly suggest a reduction in Aβ, he would have liked to see quantitation of the plaque burden using Aβ antibodies rather than just silver staining, and also more replications of the experiments, which were done the same way with only one group of mice.
How did the ultrasound treatment lighten the Aβ load? To find out, the researchers first looked to microglia, the brain’s immune cells, since some researchers believe that they mop up plaques by phagocytosis. Using confocal microscopy, Leinenga found that compared with sham-treated animals, microglia in the ultrasound-treated mice harbored twice as much Aβ in their lysosomal compartments, as measured by Aβ co-localization with the lysosomal marker CD68.
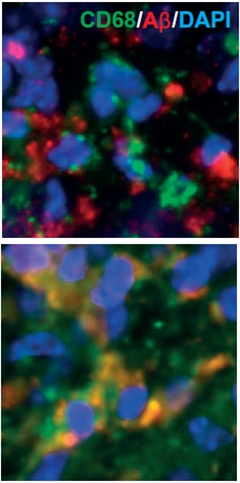
Microglial Feast?
Microglia ignore Aβ (red) in APP23 mice (top panel), but in mice treated with ultrasound, appear to take up Aβ into lysosomes (green). [Image courtesy of Leinenga and Götz, Science Translational Medicine, 2015.]
The total number of microglia—as measured by the area of Iba1+ staining—did not increase with ultrasound treatment, nor did the numbers of activated GFAP+ astrocytes. However, the researchers did find that microglial cells expressed higher levels of CD68 and took on a less branched morphology, indicating that they were somehow activated by the ultrasound treatment, and thus stimulated to engulf Aβ.
While it remains to be seen how the ultrasound treatment turned on the microglia, the researchers hypothesized that the influx of molecules from the blood could have played a role. In support of this idea, they found that treating a microglial cell line with albumin boosted the cells’ uptake of Aβ. However, albumin is only one of many blood-borne proteins that could have riled up the microglia, Götz told Alzforum.
Town agreed. “If a large protein like albumin is getting in, then we can assume many other substances from the blood are too,” he said. Town suggested that any number of these substances could affect microglia, or that some sort of mechanical deformation created by the ultrasound pulse turned them on. However, if that were the case, one might expect that astrocytes, which are intimately associated with the BBB through their endfeet, would respond as well, Town said. The researchers found no evidence of astroglial activation. “It seems that this treatment is tickling the microglia in a relatively specific fashion,” Town added.
Nixon said that the microglial response to the opening of the barrier made sense. “It’s a logical response of microglia to start sopping up the material that’s seeping in,” he said. “And if Aβ happens to be there as well, then that will be one of the substrates taken up and degraded.” However, Nixon added that while the evidence suggests microglia took up Aβ, the co-localization of lysosomal CD68 and Aβ was not sufficiently quantitative or at high enough resolution to confirm the Aβ was actually inside of lysosomes. Nixon claimed that the microglia in the ultrasound-treated mice appeared to have an overabundance of lysosomes, and that microglia ramp up lysosomal biogenesis when the lysosomal pathway is clogged or impaired. The abundance of Aβ could indicate a lysosomal traffic jam more than it indicates productive degradation, he said. Gary Landreth of Case Western Reserve University in Cleveland echoed Nixon’s concerns about the lysosomal staining. He was impressed with the widespread opening of the BBB and the reduction in plaque burden, but felt that further analysis of the activation state of plaque-associated microglial was needed (see full comment below).
Fortunately, by its very nature, ultrasound is highly tunable. At its highest intensity, it delivers shock waves powerful enough to break apart kidney stones. At lower levels, ultrasound merely heats up tissue, and clinicians use it to ablate unwanted masses such as uterine fibroids. An ongoing trial for Parkinson’s disease is testing if this thermal effect can wipe out misfiring neurons in the thalamic nucleus that cause tremors (see clinicaltrials.gov and Lipsman et al., 2013). Diagnostic ultrasound is delivered at a far lower intensity still, and the energy the researchers used to penetrate the BBB with microbubbles is just above that diagnostic level, explained Elisa Konofagou of Columbia University in New York City. Konofagou has worked over the past decade to optimize the procedure for drug delivery, and feels that it will provide a more targeted way to deliver therapy to the brain than chemical disruption of the BBB. Experiments in rhesus macaques indicate this can be done safely (see McDannold et al., 2012).
Konofagou said she was surprised when Burgess and colleagues first reported that the ultrasound could work therapeutically in AD mice without drugs, but now that Leinenga and Götz confirm and extend this finding and implicate microglia in the process, she thinks a mechanism for the phenomenon may be emerging. Konofagou said that her lab has also observed activation of microglia in response to ultrasound. “It seems like opening the barrier would be dangerous,” she said, “but it looks like cells in the brain know how to respond to such breaches.” Clearance of Aβ may therefore be a beneficial side effect of microglia responding to a barrier burglar alarm. —Jessica Shugart
References
Webinar Citations
News Citations
Research Models Citations
Paper Citations
- Vykhodtseva NI, Hynynen K, Damianou C. Histologic effects of high intensity pulsed ultrasound exposure with subharmonic emission in rabbit brain in vivo. Ultrasound Med Biol. 1995;21(7):969-79. PubMed.
- Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 2001 Sep;220(3):640-6. PubMed.
- Konofagou EE. Optimization of the ultrasound-induced blood-brain barrier opening. Theranostics. 2012;2(12):1223-37. Epub 2012 Dec 31 PubMed.
- Choi JJ, Wang S, Brown TR, Small SA, Duff KE, Konofagou EE. Noninvasive and transient blood-brain barrier opening in the hippocampus of Alzheimer's double transgenic mice using focused ultrasound. Ultrason Imaging. 2008 Jul;30(3):189-200. PubMed.
- Samiotaki G, Acosta C, Wang S, Konofagou EE. Enhanced delivery and bioactivity of the neurturin neurotrophic factor through focused ultrasound-mediated blood-brain barrier opening in vivo. J Cereb Blood Flow Metab. 2015 Jan 14; PubMed.
- Wang S, Olumolade OO, Sun T, Samiotaki G, Konofagou EE. Noninvasive, neuron-specific gene therapy can be facilitated by focused ultrasound and recombinant adeno-associated virus. Gene Ther. 2015 Jan;22(1):104-10. Epub 2014 Nov 6 PubMed.
- Burgess A, Dubey S, Yeung S, Hough O, Eterman N, Aubert I, Hynynen K. Alzheimer disease in a mouse model: MR imaging-guided focused ultrasound targeted to the hippocampus opens the blood-brain barrier and improves pathologic abnormalities and behavior. Radiology. 2014 Dec;273(3):736-45. Epub 2014 Sep 15 PubMed.
- Lipsman N, Schwartz ML, Huang Y, Lee L, Sankar T, Chapman M, Hynynen K, Lozano AM. MR-guided focused ultrasound thalamotomy for essential tremor: a proof-of-concept study. Lancet Neurol. 2013 May;12(5):462-8. Epub 2013 Mar 21 PubMed.
- McDannold N, Arvanitis CD, Vykhodtseva N, Livingstone MS. Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques. Cancer Res. 2012 Jul 15;72(14):3652-63. Epub 2012 May 2 PubMed.
External Citations
Further Reading
No Available Further Reading
Primary Papers
- Leinenga G, Götz J. Scanning ultrasound removes amyloid-β and restores memory in an Alzheimer's disease mouse model. Sci Transl Med. 2015 Mar 11;7(278):278ra33. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Indiana University School of Medicine
This is an interesting and provocative study. There have been several related studies that have generated some interest, but this work has been at the margins of most people’s expertise and the technical aspects of the ultrasound treatment are hard for a novice to evaluate.
However, the present study reports the ability to open the BBB over a broad area of cortex. It is not clear how long the treatment opens the barrier and this is a critical piece of information. The analysis of tissue damage that accompanied the ultrasound treatment is superficial and requires a more detailed examination.
The Y maze data suggests that ultrasound improves behavior in APP23 mice; however, it is not evident that they have a transgene effect in their APP23 transgenic mice, complicating the interpretation of these data. The novel object-recognition data appear sound. In summary, the behavioral data hold promise.
The plaque reduction with five treatments over six weeks is about 50 percent and these data are impressive. The Aβ peptide analysis is a bit unusual and it is not obvious why soluble Aβ levels would fall, although the effect sizes are substantial.
The most controversial aspect of this study revolves around microglial “activation.” “Activation,” a nebulous term, is not necessarily correlated with morphology and is a poor metric. For this analysis the plaque-associated macrophages/microglia need to be examined in greater detail (the movie wasn't available). The low power images are not very informative, but the CD68 and NFkB analysis is helpful. There appears to be a difference in Iba1 staining within an hour of ultrasound treatment, suggesting that the treatment elicits very rapid changes in microglia. One wonders if this is reflective of their initiation of phagocytosis. It is difficult to know what to conclude from the skeletonized microglia analysis, but I don't believe it can necessarily be interpreted as “activation.”
The argument that albumin is involved in amyloid clearance in vivo is not convincing. When the BBB opens, all serum proteins enter the brain. Many of these, most prominently complement, could support the phagocytic clearance of Aβ.
In summary, the basic phenomenology is compelling and bears further study.
View all comments by Gary LandrethHertie Institute for Clinical Brain Research, University of Tübingen, and DZNE Tübingen
This is a very innovative and interesting paper. Naturally, I would have expected that such a treatment would make the mice worse and not better. As the authors acknowledge, more work appears necessary to follow up on this provocative and novel finding.
View all comments by Mathias JuckerThe University of Queensland
We are currently doing experiments to address some of the issues that have been raised by Dr. Landreth. There is a wealth of data established in species ranging from rodents to macaques, showing that ultrasound allows for a safe opening of the BBB (some of this work has been cited—see e.g., papers by the Hynynen and Konofagou groups cited above). With the parameters chosen by us, the BBB opens transiently and selectively for a few hours and closes within one to two days. Regarding the behavioral data, the first cohort of mice was analyzed in the Y-maze. To strengthen our behavioral analysis, we conducted not only a novel object recognition test (referred to by Dr. Landreth) but also an active place-avoidance test. Regarding albumin, this serum protein is indeed only one of many factors entering the brain. Some of these have been shown to interact with Aβ, including albumin, transthyretin, and immune factors, but we chose to look at albumin first as it is the most abundant plasma protein, it is reduced in AD brains, and we were detecting its entry into the brain with Evans Blue in the ultrasound-treated mice. We are, at this stage, not claiming that albumin is the key protein in this process, we only show in vitro that albumin facilitates the uptake of amyloid, suggesting that it could play a role in vivo. The skeleton analysis complements the intensive microglial analysis and additional supporting data are shown in the supplementary movie file. We do show that amyloid is taken up by microglia into their lysosomes and our data on “cleared plaques” show that scanning ultrasound activates microglia to clear amyloid.
View all comments by Jürgen GötzMake a Comment
To make a comment you must login or register.