Subjective Memory Complaints Tied to Tau
Quick Links
Physicians often hear from their older patients, “My memory just isn’t what it used to be.” For some, this perception of mental slippage, too slight to be detected on any cognitive test, may signal early Alzheimer’s disease. Such subjective cognitive decline (SCD) remains tricky to define and measure, and the basis for it has been equally elusive. Is there a pathological process already underway in the brain, or some other less dire explanation?
- Subjective cognitive decline can be an early sign of AD, but its neurological basis is unknown.
- In PET scans of healthy old people, SCD correlated with tau in the entorhinal cortex, less so with amyloid.
- SCD may flag tau accumulation.
Two new studies look at why and when subjective changes appear, and how best to measure them. Using tau PET imaging in healthy older adults, Rebecca Amariglio and colleagues at Massachusetts General Hospital in Charlestown found that SCD correlated most strongly with the presence of neurofibrillary tangles in the entorhinal cortex, and to a lesser extent with amyloid load. The results, published on October 2 in JAMA Neurology, indicate that subjective memory complaints could be the first outward sign of tau build-up in the brain. Whether that tau deposition is a normal part of aging, or the beginnings of AD, remains to be determined.
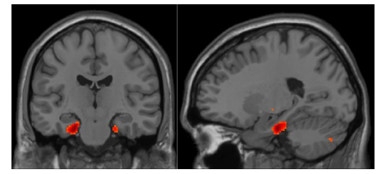
Tell-Tale Tau?
Binding of tau-PET tracer to neurofibrillary tau in the entorhinal cortex correlates with subjective cognitive decline in healthy older adults. [Courtesy of Rachel Buckley.]
Not everyone with SCD goes on to develop AD. The challenge is to figure out who will and who won’t. In a second study, Yakeel Quiroz at MGH in Boston went looking for clues in the Colombian early onset AD kindred, in which carriers of the PSEN1 Paisa mutation develop an aggressive form of early onset AD accompanied by subtle brain changes that start in childhood (Jul 2015 news). In a new study, Quiroz found that mutation carriers reported higher levels of subjective memory complaints (SMCs) than noncarriers in the decades before onset of symptoms, but SMCs did not increase with age. Thus, in this young cohort destined to develop AD, memory complaints do not escalate as disease progresses. That work appeared September 6 in Neurology.
Tau Trouble
Previous work from Amariglio’s group and others used PET to relate concerns about memory to amyloid deposition in the brain. They found the more amyloid, the more concerns (Amariglio et al., 2012; Perrotin et al., 2012). In the new study, first author Rachel Buckley expanded the work, scanning 133 healthy adults from the Harvard Aging Brain Study with the tau ligand flortaucipir, a.k.a. AV1451. The average age of participants was 76, and they were free of signs of dementia or depression. Buckley quizzed them about memory and other cognitive complaints, generating a composite score for SCD. The same people also underwent PET scanning with PiB to detect amyloid.
For tau PET, Buckley focused on two brain regions—the entorhinal and inferior temporal cortices. The former begins to accumulate tau during normal aging, which then spreads to the temporal cortex as amyloid load accrues.
The researchers found that SCD scores correlated most strongly with entorhinal tau, to a lesser extent with amyloid levels, and not at all with tau in the inferior temporal cortex. In a model that combined amyloid and tau, the latter dominated, while the amyloid contribution disappeared. “The tau effect really swamped any role for amyloid,” said Amariglio. The two did not interact, but both were independently associated with SCD. In a testament to how tricky it is to understand subjective complaint, low mood also correlated with higher SCD scores, as expected from previous work.
“This new work confirms what we’ve found in our autopsy studies,” noted Richard Kryscio, University of Kentucky, Lexington. “We have associated SCD with an excess of tangles in the medial temporal lobe, which includes the entorhinal cortex,” he told Alzforum (Kryscio et al., 2016).
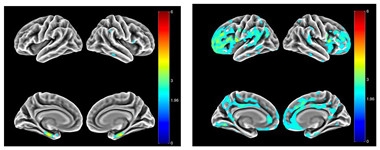
Where Trouble Starts.
Surface maps showing brain regions where tau (left) or amyloid (right) pathology associates with subjective cognitive decline. Colors show strength of correlation. [Courtesy of Rachel Buckley.]
The researchers don’t know if tau deposition and associated SCD is a consequence of aging, or early AD, but they are performing serial measurements of tau and cognition to track progression in their cohort. “We want to see who progresses to AD pathology and who doesn’t, and that might help determine which part of this tau story reflects simply aging and which doesn’t,” said Amariglio.
In the Colombian study, first author Daniel Norton assessed 26 mutation carriers and 26 noncarriers between the ages of 24 and 53 for SMCs, a measure comparable but not identical to Buckley’s SCD. None of the participants know their genetic status.
Overall, the carriers as a group showed higher SMC scores than noncarriers, suggesting that SMC does reflect early cognitive changes related to AD. However, when the investigators plotted scores as a function of age, the number of self-reported SMCs stayed steady with time in both carriers and noncarriers. In contrast, reports from close family members or caregivers of carriers increased over time, and peaked near the time of first symptoms.
Due to the small sample size, Quiroz said they cannot be sure why self-report did not increase with age, even in the face of ongoing disease progression. One possibility is that carriers’ abilities to assess their own memory difficulties diminish with time and encroaching dementia, but that remains to be proven, she said.
The data emphasize the value of partner reports over solely self-report, which has been a matter of debate in the field, said Quiroz. “In this case the study partner report is very powerful,” she told Alzforum.
While it is unclear how familial AD compares to more common late-onset, John Ringman, Keck School of Medicine, Los Angeles, said the data fit with his clinical experience. “Caregiver or family member reports are more robust in predicting the presence of AD or neurodegenerative disease,” he told Alzforum. Ringman said he also puts more weight on parameters that measure change over time, which the current study did not assess. Finally, he stressed the role for anxiety. “The study controlled for depression, but ideally they would also control for anxiety, because people are expressing worry about their mental capacities,” he said.
The underlying cause of SCD in familial AD remains to be clarified. Compared with late-onset AD, the Colombian subjects were much younger and less likely to have age-related tau deposition. In the DIAN study of autosomal-dominant AD, subjects remained mainly negative for tau by PET scans until disease onset, after which tau increased quickly (Aug 2017 conference news). In the Colombian families, Quiroz said she saw increased tau levels in some carriers six years from clinical onset, raising the possibility that tau may factor into SMC in this cohort (Feb 2016 conference news).
Together, the two studies highlight a challenge facing researchers who study subjective changes, said Christoph Laske, University Hospital of Tübingen in Germany. “That both papers are using different terms (SCD versus SMC) and different quantitative measures for subjective memory problems demonstrates the urgent need to better standardize this research topic in the near future," Laske wrote in an email to Alzforum.—Pat McCaffrey
References
News Citations
- Familial Alzheimer’s Gene Alters Children’s Brains
- Data from DIAN Revise Familiar Biomarker Trajectories
- Tau Tracers Track First Emergence of Tangles in Familial Alzheimer’s
Paper Citations
- Amariglio RE, Becker JA, Carmasin J, Wadsworth LP, Lorius N, Sullivan C, Maye JE, Gidicsin C, Pepin LC, Sperling RA, Johnson KA, Rentz DM. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia. 2012 Oct;50(12):2880-6. PubMed.
- Perrotin A, Mormino EC, Madison CM, Hayenga AO, Jagust WJ. Subjective cognition and amyloid deposition imaging: a Pittsburgh Compound B positron emission tomography study in normal elderly individuals. Arch Neurol. 2012 Feb;69(2):223-9. PubMed.
- Kryscio RJ, Abner EL, Jicha GA, Nelson PT, Smith CD, Van Eldik LJ, Lou W, Fardo DW, Cooper GE, Schmitt FA. Self-Reported Memory Complaints: A Comparison of Demented and Unimpaired Outcomes. J Prev Alzheimers Dis. 2016 Mar;3(1):13-19. PubMed.
Other Citations
Further Reading
Papers
- Laske C, Sohrabi HR, Jasielec MS, Müller S, Koehler NK, Gräber S, Förster S, Drzezga A, Mueller-Sarnowski F, Danek A, Jucker M, Bateman RJ, Buckles V, Saykin AJ, Martins RN, Morris JC, Dominantly Inherited Alzheimer Network Dian. Diagnostic Value of Subjective Memory Complaints Assessed with a Single Item in Dominantly Inherited Alzheimer's Disease: Results of the DIAN Study. Biomed Res Int. 2015;2015:828120. Epub 2015 Apr 2 PubMed.
Primary Papers
- Buckley RF, Hanseeuw B, Schultz AP, Vannini P, Aghjayan SL, Properzi MJ, Jackson JD, Mormino EC, Rentz DM, Sperling RA, Johnson KA, Amariglio RE. Region-Specific Association of Subjective Cognitive Decline With Tauopathy Independent of Global β-Amyloid Burden. JAMA Neurol. 2017 Dec 1;74(12):1455-1463. PubMed.
- Ossenkoppele R, Jagust WJ. The Complexity of Subjective Cognitive Decline. JAMA Neurol. 2017 Dec 1;74(12):1400-1402. PubMed.
- Norton DJ, Amariglio R, Protas H, Chen K, Aguirre-Acevedo DC, Pulsifer B, Castrillon G, Tirado V, Munoz C, Tariot P, Langbaum JB, Reiman EM, Lopera F, Sperling RA, Quiroz YT. Subjective memory complaints in preclinical autosomal dominant Alzheimer disease. Neurology. 2017 Oct 3;89(14):1464-1470. Epub 2017 Sep 6 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Eisai Inc.
Due to inconsistent results, it is still under debate whether subjective cognitive decline (SCD) represents an early sign of Alzheimer’s disease. Previous evidence showed that SCD was associated with future objective cognitive decline and conversion from normal cognition to mild cognitive impairment. More recently, studies on SCD showed brain abnormalities related to AD, such as increased cerebral Aβ deposition, greater atrophy and glucose hypometabolism, and disrupted functional connectivity changes.
The major issues that prevent the use of SCD as an early indicator of AD are related to the variability found in the literature and the lack of clear neurobiological mechanisms underlying SCD.
In this JAMA Neurology paper, Buckley and colleagues take a step forward in understanding the neurobiological mechanisms underlying SCD by using PET flortaucipir F 18 to detect paired helical filaments of tau in the entorhinal cortex and the inferior temporal region, and Carbon 11–labeled Pittsburgh compound B to determine the global Aβ burden. Results from the linear regression model (controlling for age, sex, educational attainment, and Geriatric Depression Scale score) showed that greater SCD was associated with increasing entorhinal cortical tau burden and Aβ burden but not inferior temporal tau burden.
The second study, by Norton and colleagues, investigated the frequencies of self-reported and partner-reported subjective memory complaints in individuals from a Colombian kindred with high prevalence of the presenilin-1 (PSEN-1) E280A mutation, and their association with age and hippocampal volume. Results revealed higher self-reported subjective memory complaints in carriers compared to noncarriers. Moreover, informant-reported subjective memory complaints and hippocampal volume significantly related to age, while self-reported subjective memory complaints did not. However, a previous study on the same population did not find any difference between cognitively unimpaired carriers and noncarriers, indicating that further studies should be conducted in order to clarify the role of subjective memory complaints in early onset AD.
These manuscripts highlight new evidence on the genetic and pathophysiological bases of SCD. The definition of SCD is still evolving and the association between SCD and depressive symptoms is not clear yet. Moreover, it is not clear how the results found in these populations might be more related to depressive symptoms than to AD pathology. Finally, the impact of age in the neurodegeneration processes and its association with SCD is still under debate, as shown by the results of Norton and colleagues. Future studies investigating whether SCD might represent an early marker of AD should take into account depressive symptoms and age as independent factors, not exclusively as covariate, in order to understand exclusively the impact of SCD in relation to AD and to exclude neurodegeneration processes due to age or depressive symptoms. SCD might represent the early clinical expression of neurodegeneration during aging and depression.
References:
Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatr Scand. 2014 Dec;130(6):439-51. Epub 2014 Sep 13 PubMed.
Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chételat G, Dubois B, Dufouil C, Ellis KA, van der Flier WM, Glodzik L, van Harten AC, de Leon MJ, McHugh P, Mielke MM, Molinuevo JL, Mosconi L, Osorio RS, Perrotin A, Petersen RC, Rabin LA, Rami L, Reisberg B, Rentz DM, Sachdev PS, de la Sayette V, Saykin AJ, Scheltens P, Shulman MB, Slavin MJ, Sperling RA, Stewart R, Uspenskaya O, Vellas B, Visser PJ, Wagner M, Subjective Cognitive Decline Initiative (SCD-I) Working Group. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014 May 3; PubMed.
La Joie R, Perrotin A, Egret S, Pasquier F, Tomadesso C, Mézenge F, Desgranges B, de La Sayette V, Chételat G. Qualitative and quantitative assessment of self-reported cognitive difficulties in nondemented elders: Association with medical help seeking, cognitive deficits, and β-amyloid imaging. Alzheimers Dement (Amst). 2016;5:23-34. Epub 2016 Dec 18 PubMed.
Lista S, Molinuevo JL, Cavedo E, Rami L, Amouyel P, Teipel SJ, Garaci F, Toschi N, Habert MO, Blennow K, Zetterberg H, O'Bryant SE, Johnson L, Galluzzi S, Bokde AL, Broich K, Herholz K, Bakardjian H, Dubois B, Jessen F, Carrillo MC, Aisen PS, Hampel H. Evolving Evidence for the Value of Neuroimaging Methods and Biological Markers in Subjects Categorized with Subjective Cognitive Decline. J Alzheimers Dis. 2015 Sep 24;48 Suppl 1:S171-91. PubMed.
Ossenkoppele R, Jagust WJ. The Complexity of Subjective Cognitive Decline. JAMA Neurol. 2017 Dec 1;74(12):1400-1402. PubMed.
Buckley RF, Hanseeuw B, Schultz AP, Vannini P, Aghjayan SL, Properzi MJ, Jackson JD, Mormino EC, Rentz DM, Sperling RA, Johnson KA, Amariglio RE. Region-Specific Association of Subjective Cognitive Decline With Tauopathy Independent of Global β-Amyloid Burden. JAMA Neurol. 2017 Dec 1;74(12):1455-1463. PubMed.
Norton DJ, Amariglio R, Protas H, Chen K, Aguirre-Acevedo DC, Pulsifer B, Castrillon G, Tirado V, Munoz C, Tariot P, Langbaum JB, Reiman EM, Lopera F, Sperling RA, Quiroz YT. Subjective memory complaints in preclinical autosomal dominant Alzheimer disease. Neurology. 2017 Oct 3;89(14):1464-1470. Epub 2017 Sep 6 PubMed.
Lopera F, Ardilla A, Martínez A, Madrigal L, Arango-Viana JC, Lemere CA, Arango-Lasprilla JC, Hincapíe L, Arcos-Burgos M, Ossa JE, Behrens IM, Norton J, Lendon C, Goate AM, Ruiz-Linares A, Rosselli M, Kosik KS. Clinical features of early-onset Alzheimer disease in a large kindred with an E280A presenilin-1 mutation. JAMA. 1997 Mar 12;277(10):793-9. PubMed.
University Hospital of Tübingen
The paper by Buckley et al. shows that higher subjective cognitive decline (SCD) scores are associated with higher tau burden in the brain in clinically healthy older adults. However, this finding does not yet justify considering SCD as an indicator for tauopathy in the brain, as we get no information about diagnostic accuracy, sensitivity, specificity, positive predictive value, and negative predictive value of SCD for the presence of tauopathy in the brain. In addition, higher tau levels in the brain are not necessarily pathological levels.
Norton and colleagues demonstrate that cognitively normal carriers of the PSEN-1 E280A mutation show higher subjective memory complaint (SMC) scores than noncarriers. This indicates that increased SMC scores seem to be an early sign of autosomal-dominant Alzheimer`s disease (ADAD). However, the information about estimated years to symptom onset is missing, so it is not possible to say how early SMC scores are rising in the preclinical stage of ADAD.
That both papers are using different terms (SCD versus SMC) and different quantitative measures for subjective memory problems demonstrates the urgent need to better standardize this research topic in the near future.
Make a Comment
To make a comment you must login or register.