Synaptic Genes Determine Brain Connectivity
Quick Links
Coordinated activity within brain networks falters early in Alzheimer’s disease and other disorders. The strength of these network connections can be inherited, suggesting this trait has a genetic basis. But what genes actually determine the robustness of connections between remote brain regions? In the June 12 Science, researchers led by Michael Greicius at Stanford University in California describe a new way to address this question. They compared gene expression in four well-studied networks of human brain, finding 136 genes that varied in tandem across all the networks. The set was enriched for synaptic genes, such as ion channels and receptors. Polymorphisms in these genes correlated with weaker or stronger connectivity of the four networks in healthy young people, and also associated with Alzheimer’s and other neurological diseases, implying these genes can alter communication along axonal pathways. “The data convinced us that there are strong molecular underpinnings to these measurements of large-scale brain networks,” Greicius told Alzforum. “This approach allows us to bridge the gap between systems-level and molecular-level investigations.”
Jeremy Miller at the Allen Institute for Brain Science in Seattle praised the strategy of combining imaging, measures of connectivity, and genetics into a single, straightforward story. “This is one of the first papers to show gene expression similarities between distant cortical regions,” he wrote to Alzforum. Miller was not involved in the research, but has worked with one of the co-authors in the past.
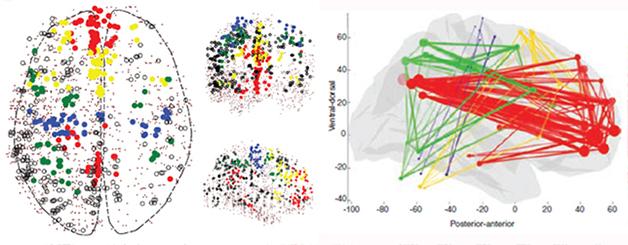
Making Connections. Regions belonging to four networks (red=DMN, yellow=salience, green=visuospatial, blue=sensorimotor) coordinate expression of a set of genes (left panel). Variants in these genes associate with stronger connections within each network (right panel). [Courtesy of Science/AAAS.]
Brain networks can be identified during resting-state functional MRIs, when volunteers lie in the scanner without performing any specific task. Over a timescale of seconds, activity in widely distributed brain regions waxes and wanes in concert, revealing the existence of several functional networks (see May 2007 news). Network firing becomes disorganized early in AD and other neurological conditions (see Nov 2007 news; Jul 2012 news; Aug 2013 news).
Intriguingly, the strength of brain connectivity can be inherited (see Glahn et al., 2010; Fornito et al., 2011). In particular, variations in the Alzheimer’s genes ApoE and clusterin and the frontotemporal dementia gene progranulin have been linked to weaker connectivity (see Dec 2010 news; Dec 2011 news; Dec 2013 news). For the most part, however, the genes involved in this trait have remained mysterious.
Joint first authors Jonas Richiardi and Andre Altmann turned up new genes by first collecting resting-state fMRI data from 15 healthy young adults. This data defined the boundaries of specific brain regions involved in each of four non-overlapping networks: the dorsal default mode, salience, sensorimotor, and visuospatial. The authors then compared gene expression across all the brain regions involved using the Allen Institute’s microarray dataset generated from six healthy adults who donated their brains postmortem. They found a set of 136 genes that varied their expression in common across all four networks. Ion channels and neurotransmitters predominated on this list. “That was not a big surprise, but it was reassuring,” Greicius told Alzforum. “These are the sort of genes we would expect to see if functional connectivity is a real neural phenomenon.”
To validate a role for these genes in connectivity, the authors compared genetic and resting-state fMRI data from 259 healthy adolescents in the IMAGEN database, a European project that studies risk-taking behavior in teenagers. In this cohort, genetic polymorphisms associated with differences in connectivity. Next, the authors turned to the Allen Mouse Brain Connectivity Atlas, which maps axonal connections between brain regions. Among physically connected regions, a set of 57 mouse orthologs of the human connectivity genes demonstrated a similar expression pattern, but sets of random genes did not. The finding tied these synaptic genes to structural as well as functional connectivity.
Finally, the authors entered the gene list into the Database for Annotation, Visualization, and Integrated Discovery (DAVID), which revealed links between the overall gene set and several neurological diseases, including Alzheimer’s, Huntington’s, amyotrophic lateral sclerosis, and schizophrenia. The initial analysis did not pinpoint specific genes for each condition, but Greicius plans to dig deeper in future studies. He noted that Alzheimer’s disease is closely associated with a loss of connectivity in the default mode network (DMN) in particular. Genes that exclusively promote connectivity within the DMN thus might associate with Alzheimer’s risk. Likewise, disruptions in the salience network are associated with frontotemporal dementia, so study of this network might suggest new FTD genes (see Zhou et al., 2010; Lee et al., 2014).
Bruce Yankner at Harvard Medical School was intrigued by the similarity in synaptic gene expression along interconnected neural networks. The finding raises the question of which came first, he wrote to Alzforum. Do common gene expression patterns give rise to connectivity, or does connectedness and coordinated excitation determine gene expression? The second possibility seems most plausible, and could be tested by using optogenetic control of neurons in animal models to investigate whether forced changes in firing patterns alter the expression of the common gene set, he suggested.—Madolyn Bowman Rogers
References
News Citations
- Boston: Resting State MRI Shows Loss of Network Connectivity Early in AD
- Functional Imaging Gives Early Glimpse of AD
- Communication Breakdown: Multiple Networks Decline in AD Brains
- Brain Connectivity Reveals Preclinical Alzheimer’s Disease
- A Foreshadowing? ApoE4 Disrupts Brain Connectivity in Absence of Aβ
- Neuroimaging Offers a CLU to AD Risk Factor’s Functional Effects
- Risk Genes Influence Brain Connectivity in Preclinical FTD
Paper Citations
- Glahn DC, Winkler AM, Kochunov P, Almasy L, Duggirala R, Carless MA, Curran JC, Olvera RL, Laird AR, Smith SM, Beckmann CF, Fox PT, Blangero J. Genetic control over the resting brain. Proc Natl Acad Sci U S A. 2010 Jan 19;107(3):1223-8. PubMed.
- Fornito A, Zalesky A, Bassett DS, Meunier D, Ellison-Wright I, Yücel M, Wood SJ, Shaw K, O'Connor J, Nertney D, Mowry BJ, Pantelis C, Bullmore ET. Genetic influences on cost-efficient organization of human cortical functional networks. J Neurosci. 2011 Mar 2;31(9):3261-70. PubMed.
- Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, Kramer JH, Weiner M, Miller BL, Seeley WW. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer's disease. Brain. 2010 May;133(Pt 5):1352-67. PubMed.
- Lee SE, Khazenzon AM, Trujillo AJ, Guo CC, Yokoyama JS, Sha SJ, Takada LT, Karydas AM, Block NR, Coppola G, Pribadi M, Geschwind DH, Rademakers R, Fong JC, Weiner MW, Boxer AL, Kramer JH, Rosen HJ, Miller BL, Seeley WW. Altered network connectivity in frontotemporal dementia with C9orf72 hexanucleotide repeat expansion. Brain. 2014 Nov;137(Pt 11):3047-60. Epub 2014 Oct 1 PubMed.
External Citations
Further Reading
News
- ApoE4 Linked to Default Network Differences in Young Adults
- Boosting Memory Through Stimulation of a Brain Network
- Neural Circuitry Goes Haywire in Both Sporadic and Familial AD
- Cognitive Deficits Arise From Network, Not Regional, Dysfunction
- Atrophy of Distinct Brain Networks May Explain Alzheimer’s Variants
- Wired for Memory? Electrical and Structural Connectivity Linked
Primary Papers
- Richiardi J, Altmann A, Milazzo AC, Chang C, Chakravarty MM, Banaschewski T, Barker GJ, Bokde AL, Bromberg U, Büchel C, Conrod P, Fauth-Bühler M, Flor H, Frouin V, Gallinat J, Garavan H, Gowland P, Heinz A, Lemaître H, Mann KF, Martinot JL, Nees F, Paus T, Pausova Z, Rietschel M, Robbins TW, Smolka MN, Spanagel R, Ströhle A, Schumann G, Hawrylycz M, Poline JB, Greicius MD, IMAGEN consortium. Correlated gene expression supports synchronous activity in brain networks. Science. 2015 Jun 12;348(6240):1241-4. Epub 2015 Jun 11 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.