Synuclein Oligomers: Is EnSNAREing Synaptic Vesicles Their True Calling?
Quick Links
Alpha-synuclein is a card-carrying member of the infamous Lewy body aggregates which mark Parkinson’s disease and other synucleinopathies. These aggregates are thought to form when α-synuclein oligomerizes. Now, two studies contend that some α-synuclein oligomers may also serve an important physiological function. Writing in the September 25 Current Biology, researchers led by Subhojit Roy at the University of California, San Diego, report that the protein multimers latch onto synaptic vesicles, herding them together so they won’t be recycled. Another study, led by 2013 Nobel Laureate Thomas Südhof at Stanford University in California, found that α-synuclein multimerizes upon binding to membranes, and recruits protein complexes called SNAREs that dock vesicles to the presynaptic terminal. Südhof’s study appeared September 22 in the Proceedings of the National Academy of Sciences. Both studies tout α-synuclein multimers as synaptic vesicle wranglers. They also raise questions about α-synuclein’s role in neurotransmission, and about what may tip the balance from functional oligomers to toxic aggregates.
“I was very excited to see these papers,” commented Quyen Hoang of Indiana University in Indianapolis. “They get us closer to understanding what this protein does, as well as the conformational changes that occur when it performs its function.” Hoang was co-author on one of two controversial papers that reported that α-synuclein, long believed to have little secondary structure, forms stable tetramers (see Wang et al., 2011).
“These interesting papers clearly report the existence of physiological α-syn multimers, whereas only a few years ago, α-syn assemblies—whether in cytoplasm or at membranes—had been interpreted as pathological,” Ulf Dettmer, Tim Bartels, and Dennis Selkoe of Brigham and Women’s Hospital, Boston, wrote in a joint comment to Alzforum.
Researchers have waged a spirited debate about the nature of α-synuclein’s physiological forms, and which of them beget toxic aggregates. Many believe that the protein primarily exists as an unfolded monomer (see Feb 2012 news story), while others, including Hoang and Selkoe, postulate that it forms a stable tetramer (see Aug 2011 news story). However, while α-synuclein aggregates are primarily cytoplasmic, the protein’s physiological milieu is thought to be the presynaptic terminal, where it associates with synaptic vesicles and abounds in various shapes and sizes (see Feb 2014 news story).
In vitro studies have reported that α-synuclein clusters liposomes. It promotes the formation of the well-studied soluble N-ethylmaleimide-sensitive factor attachment protein complexes—aka SNAREs—which tether vesicles to the plasma membrane prior to their release into the synapse. However, in what form and to what end α-synuclein binds to vesicles have been mysteries. Alpha-synuclein’s physiological role in the synapse is the key to understanding how it causes disease. “You have to understand the normal to understand the abnormal,” Roy told Alzforum.
In Roy’s Current Biology study, first author Lina Wang and colleagues monitored the multimerization of α-synuclein within synapses in cell culture. They used a two-part reporter system in which α-synuclein was tagged with either the N- or C-terminal half of the Venus fluorescent protein. When α-synuclein molecules get together, these two halves (VN/VC) reconstitute and the protein fluoresces. Once associated, the tags stick together, allowing the visualization of interactions that otherwise would be transient. Dimers and potentially larger multimers accumulate as a result. The researchers expressed the tagged α-synuclein at near-normal levels in primary neurons from α-synuclein knockout mice. Four hours after transfection, fluorescent α-synuclein started to appear in the synapses, where it grew more concentrated over time.

Synaptic Hook-up.
Alpha-synuclein complexes (green) co-localize (yellow) with synaptic boutons (red) in hippocampal neurons expressing VN/VC-labeled α-synuclein, which only fluoresces when two molecules dimerize. [Image courtesy of Wang et al., Current Biology 2014.]
To zero in on where chimeric α-synuclein congregated, the researchers used fluorescent microscopy to get a detailed look at the synapse. They found that fluorescent α-synuclein occupied only a portion of the synaptic bouton. It co-localized with the synaptic vesicle marker synaptophysin. Although the VN/VC method cannot prove oligomerization beyond dimers, the researchers found that larger multimers had indeed formed when they analyzed neuronal extracts on western blots. They reported multimers in cytosolic extracts and also bound to isolated synaptic vesicle membranes.
Prompted by previous reports that α-synuclein restricts the motility of vesicles in yeast and in vitro (see Scott et al., 2012; Soper et al., 2008; and Diao et al., 2013), the researchers next asked whether multimerized α-synuclein could do the same in neurons. They transfected α-synuclein knockout neurons with VN/VC-tagged α-synuclein. They zapped these cells with an electrical pulse, a stimulus known to scatter vesicles. Vesicles in control cells dispersed. Vesicles in cells expressing VN/VC-tagged α-synuclein largely stayed together, suggesting that α-synuclein complexes corralled them.
Based on these results, Wang hypothesized that disrupting α-synuclein multimerization would reduce the clustering of vesicles. To test this, the researchers turned to untagged protein. They mutated six amino acids thought to promote multimerization. Unlike wild-type α-synuclein, this mutant failed to impede vesicle dispersion.
Some commentators, including Hoang, cautioned that the VN/VC tagging of α-synuclein could produce artifacts, but they also called the experiments carefully done. “This fluorescence complementation assay could potentially introduce false-positive associations, but with all the controls that they have done, the result seems to be quite convincing,” Hoang said.
Other commentators were impressed that Roy used intact cells to study α-synuclein multimerization. “They place the issue directly into a physiological context at the synapse, using a set of sophisticated in situ fluorescent assays,” wrote Dettmer, Selkoe, and Bartels. “It is the first paper that suggests a physiological function for the recently recognized α-synuclein multimers.”
Multimers, Membranes, and SNAREs
In Südhof’s PNAS paper, first author Jacqueline Burré and colleagues report a different approach to probing α-synuclein’s lifestyle at the synapse. Before asking whether synaptic α-synuclein existed as rogue monomers or ran in multimeric herds, the researchers focused on where α-synuclein resided, and with which partners it associated. They started by measuring its relationship with SNARE complexes, because α-synuclein had been reported to help form those complexes (see May 2010 news story). Burré started with co-immunoprecipitation experiments to look for protein-protein interactions. In vitro, the group mixed fragments of the plasma-membrane SNARE proteins syntaxin and SNAP-25 with the vesicle protein synaptobrevin, along with liposomes and α-synuclein. SNARE assembly required liposomes, and α-synuclein greatly enhanced this by binding to both synaptobrevin and liposomes.
Finding that α-synuclein latched onto both membranes and SNAREs, the researchers next wanted to see whether it would snag synaptic vesicles, and if so, if it had a preference for vesicles that floated free in the cytosol or those already tethered to the membrane. To find out, they separated synaptic vesicles from mouse brain homogenates over a sucrose gradient into 35 fractions. They stained each fraction for different SNARE proteins and α-synuclein. They only detected α-synuclein in fractions that contained both vesicle proteins and plasma membrane SNARE proteins. The authors suggested in the paper that vesicles in these fractions were docked to the plasma membrane, indicating that α-synuclein only associates with docked vesicles, not free vesicles, in the synapse.
However, Roy noted that Burré found a preponderance of “docked” vesicles. This contradicts the consensus in the field that only a small fraction of synaptic vesicles are docked at any given time. Burré responded that the fractions could contain not only docked vesicles but all vesicles within the synaptic active zone—a mesh of cytoskeletal proteins that holds vesicles near the synapse, but not necessarily attached to it. Therefore, not all vesicles in these fractions were necessarily docked at the membrane.
Poul Henning Jensen of Aarhus University in Denmark found α-synuclein’s association with synaptic vesicles very interesting. However, he noted that the researchers did not determine in this experiment whether the α-synuclein in these fractions existed as monomers or multimers.
To find out which form of α-synuclein associates with membranes, the researchers cross-linked proteins in mouse brain extracts and looked on western blots for α-synuclein species of different sizes. As they ramped up cross-linker concentrations, the amount and size of α-synuclein multimers on the blots grew. Importantly, multimers only formed in the presence of membranes. The researchers detected dimers, tetramers, octamers, and higher-order multimers in both whole brain extracts and in brain slices cross-linked prior to extraction. They concluded that the predominant species were octamers or higher, yet they could not rule out the co-existence of tetramers and dimers, which are likely the building blocks of higher-order multimers. The researchers then used fluorescence resonance energy transfer (FRET)—a technique that detects interactions between proteins—to determine that in the presence of liposomes, α-synuclein multimerizes in a highly ordered, anti-parallel stack.
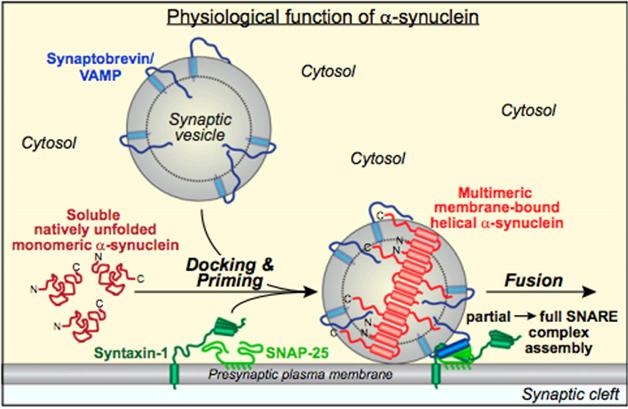
Synuclein enSNAREs Vesicles. Proposed model in which α-synuclein multimerizes upon binding to synaptic vesicles and facilitates the formation of SNARE complexes. [Image courtesy of Burré et al., PNAS 2014.]
The findings suggest that myriad multimers associate only with membranes. This conclusion seemingly contradicts that of Selkoe and others who identified α-synuclein tetramers in the cytosol. Selkoe, Bartels, and Dettmer attributed this difference to Burré’s reliance on in vitro cross-linking, saying that technique promotes the dissociation of tetramers. Selkoe and colleagues found tetramers by cross-linking α-synuclein in isolated cells rather than brain slices.
Burré’s findings revealed that α-synuclein multimerizes efficiently on membranes in extracts, but not necessarily on synaptic vesicles in vivo, commented Jensen. This is important, he said, because true synaptic vesicles are packed full of other proteins (see May 2014 news story), making it hard to imagine where large α-synuclein multimers would fit in.
Burré agreed, but speculated that the protein must fit in somewhere because it is well established that α-synuclein associates with synaptic vesicles. Furthermore, she speculated that free α-synuclein could tip the balance toward toxicity in situations where α-synuclein is overexpressed, such as in people with multiplications of the gene. “You could imagine that there is only very limited membrane area for α-synuclein to bind crowded vesicles, so an overabundance could shift equilibrium towards the cytosolic population,” she said. “Perhaps this cytosolic population may be more prone to aggregation.”
How do these two papers fit together? Burré expressed enthusiasm about Roy’s finding that α-synuclein clusters vesicles, especially since a previous study she took part in with Alex Brunger’s lab at Stanford University had shown that α-synuclein could cluster liposomes in an in vitro system (see Diao et al., 2013). “I was excited that they could see something like this in true synaptic vesicles,” she said. Burré also favors a model where α-synuclein multimers pull vesicles together. This would boost the local concentration of SNARE proteins and promote SNARE complex formation. Roy agreed that the two papers’ results could converge on such a model.
How would such vesicle clustering and SNARE complex formation ultimately affect neurotransmission? According to Roy, the preponderance of evidence suggests that α-synuclein attenuates neurotransmission, so any model would need to fit into that framework.
Said Burré, “What we consistently see is that α-synuclein promotes neurocomplex assembly, whereas other groups see that it prevents neurotransmitter release. It remains to be seen how these two actually go together.”—Jessica Shugart
References
News Citations
- Synuclein—Researchers Out of Sync on Structure
- An α-Synuclein Twist—Native Protein a Helical Tetramer
- Alpha-Synuclein Types Congregate in Presynapse—Which Is the Bad One?
- α-Synuclein’s Day Job: To Chaperone SNARE Complexes?
- Synaptic Metropolis—Microscopic Census Documents 300,000 Protein Inhabitants
Paper Citations
- Wang W, Perovic I, Chittuluru J, Kaganovich A, Nguyen LT, Liao J, Auclair JR, Johnson D, Landeru A, Simorellis AK, Ju S, Cookson MR, Asturias FJ, Agar JN, Webb BN, Kang C, Ringe D, Petsko GA, Pochapsky TC, Hoang QQ. A soluble α-synuclein construct forms a dynamic tetramer. Proc Natl Acad Sci U S A. 2011 Oct 25;108(43):17797-802. Epub 2011 Oct 17 PubMed.
- Scott D, Roy S. α-Synuclein inhibits intersynaptic vesicle mobility and maintains recycling-pool homeostasis. J Neurosci. 2012 Jul 25;32(30):10129-35. PubMed.
- Soper JH, Roy S, Stieber A, Lee E, Wilson RB, Trojanowski JQ, Burd CG, Lee VM. Alpha-synuclein-induced aggregation of cytoplasmic vesicles in Saccharomyces cerevisiae. Mol Biol Cell. 2008 Mar;19(3):1093-103. PubMed.
- Diao J, Burré J, Vivona S, Cipriano DJ, Sharma M, Kyoung M, Südhof TC, Brunger AT. Native α-synuclein induces clustering of synaptic-vesicle mimics via binding to phospholipids and synaptobrevin-2/VAMP2. Elife. 2013;2:e00592. PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Burré J, Sharma M, Südhof TC. α-Synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation. Proc Natl Acad Sci U S A. 2014 Oct 7;111(40):E4274-83. Epub 2014 Sep 22 PubMed.
- Wang L, Das U, Scott DA, Tang Y, McLean PJ, Roy S. α-synuclein multimers cluster synaptic vesicles and attenuate recycling. Curr Biol. 2014 Oct 6;24(19):2319-26. Epub 2014 Sep 25 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
U.K. Dementia Research Institute at University College London
Harvard Medical School, Brigham and Women’s Hospital
Co-Director, Brigham and Women's Hospital's Ann Romney Center for Neurologic Diseases
Wang et al. present a compelling new model for the physiological role of αSynuclein multimerization in synaptic vesicle recycling and thus neural function. Starting from the key question, “What is the form of αSyn at synapses, one of its normal locales?,” the study builds upon the recently discovered evidence that this amphipathic protein forms metastable, partially α-helical tetramers and related multimers under physiological conditions in healthy cells (Bartels et al., 2011; Dettmer et al., 2013; Westphal and Chandra, 2013; Gould et al., 2014). Wang et al. place the issue directly into a physiological context at the synapse, using a set of sophisticated in situ fluorescent assays. In their key experiment, they transduced αSyn -/- neurons with AAV particles encoding Venus YFP complementation pairs VN/VC-αSyn and visualized the entry of newly synthesized proteins into presynaptic boutons four to five hours later. They observed strong YFP signals (indicative of at least two αSyn molecules interacting) that co-localized with synaptic vesicle clusters. Then, the authors show that the stabilized αSyn multimers attenuate the activity-dependent dispersion of synaptic vesicles and suggest that αSyn multimers associate with synaptic vesicle clusters to restrict their trafficking.
The study is important in several ways. It is the first paper that suggests a physiological function for the recently recognized αSyn multimers in cells. The suggested role is generally in line with earlier studies suggesting such a function for αSyn but without addressing the assembly state of the protein. Moreover, it is the first paper that explicitly interprets oligomerization of αSyn, which has been reported in prior bimolecular fluorescence complementation studies, in light of the emerging concept of physiological multimers. Heretofore, investigators using fluorescence protein complementation to study αSyn have interpreted fluorescent signals as indicating the formation of toxic oligomers. Yet there has been doubt about this because the complementation depends on a defined position of the tags relative to αSyn and occurs very quickly (within hours) in healthy cultured cells, and even in primary neurons, as shown by Wang et al. for αSyn expressed at roughly endogenous levels (not overexpressed, as the authors emphasize). Moreover, the YFP complementation pairs (VN-αSyn and αSyn-VC) rapidly reconstitute fluorescent signals that are almost as strong as those achieved by expression of full-length Venus YFP-tagged αSyn, whereas the generation of toxic, β-sheet oligomers in acute cell culture is generally very hard to achieve. We therefore believe that αSyn fluorescent protein complementation is primarily useful to study the physiological propensity of this abundant neuronal protein to self-interact.
We expect that the findings of Wang et al. may lead to a general concept of how normal multimerization in the synuclein family (α, β, and γ) may regulate membrane homeostasis in cell types other than neurons, especially erythrocytes and their cellular precursors, which are rich in αSyn. Importantly, αSyn fluorescent protein complementation can be observed easily in cultured cells, as shown by Wang et al. (for HEK cells) and in earlier papers, including a cytosolic staining pattern consistent with soluble αSyn multimers (predominantly tetramers) that we (Bartels et al., 2011) and others (Westphal and Chandra, 2013) have observed upon multi-step purification of αSyn from red blood cells under non-denaturing conditions, and also upon differential centrifugation after live-cell crosslinking of proteins in neurons and other healthy cells (Dettmer et al., 2013). Future studies should address in detail the changes in αSyn conformation and assembly state that are associated (in neurons) with its transport to synapses, its binding to synaptic membranes/vesicles, and its release from these vesicles into the cytosol. We postulate that αSyn multimers can remain intact, at least for a time, upon dissociation from membranes, and the Wang et al. study is not incompatible with that idea.
The new study by Burré et al. is consistent with the principal finding of Wang et al. that αSyn multimers exist on membrane vesicles. Burré et al. also address the assembly size of αSyn on neuronal membranes in the context of the strong and direct Synaptobrevin-2/αSyn interaction they have proposed previously (Burré et al., 2010). Upon adding the crosslinking agents glutaraldehyde, dimethyl suberimidate (DMS), dimethyl adipimidate (DMA), and dimethyl pimelimidate (DMP) to mouse brain homogenates made in the absence of detergent, the authors find substantial amounts of αSyn in multimeric states—dimers, tetramers, and higher-order multimers (their Fig. 2). The same crosslinking only yielded monomers when the PBS-soluble (cytosolic) fraction was analyzed, prompting the authors to conclude that the multimers are entirely on membranes and not in the cytosol. Unfortunately, the relative abundance of αSyn in membrane vs. cytosol fractions in their starting total homogenates was not described. Given the well-documented cytosolic localization of ~90 percent of total cellular αSyn (as these authors themselves published [Burré et al., 2013, Fig. 1a]) and the abundance of αSyn multimers in total brain homogenates (their Fig. 2), the total absence of multimers they now report in the cytosol fraction is surprising (but see below). Also, their postulated strong and direct interaction of αSyn and synaptobrevin-2 was not probed in the crosslinked samples, although it would have been interesting to see here whether a trapping of the two proteins in a hetero-oligomer could be achieved. As a next step, they applied crosslinkers to acute brain slices, and >40 percent of αSyn was trapped at dimeric or tetrameric positions by glutaraldehyde, while the monomer pool was virtually completely depleted. This finding is only compatible with their conclusion that cytosolic αSyn is entirely monomeric if one assumes that very little αSyn in the brain is localized in the cytosol. Here, the authors missed the opportunity of defining the localization of the multimers by generating sequential extracts of the tissue (e.g., PBS->Triton->SDS; as described in Dettmer et al., 2013). (Parenthetically, we have recently done such an analysis using the well-established crosslinker disuccinimidyl glutarate [DSG] on a fresh, normal human brain biopsy [Dettmer et al., under review] and found abundant amounts of 60 kDa [tetrameric] αSyn [plus related minor 80 and 100 kDa multimers] in the PBS-soluble fraction, while the Triton fraction only contained monomers.) As their next step, and generally consistent with the αSyn fluorescence complementation done by Wang et al., Burré et al. used FRET to reveal an ordered assembly of the αSyn subunits in the oligomers. Then, using separation of membranes by a sucrose gradient, they find αSyn to be associated with docked vesicles at the presynaptic plasma membrane, in line with the finding of Wang and colleagues.
All in all, both of these interesting papers clearly report the existence of physiological αSyn multimers, whereas only a few years ago, αSyn assemblies—whether in cytoplasm or at membranes—had been interpreted as pathological, since the native form was believed to be an unfolded monomer. The strength of the study by Wang et al. is their almost exclusive use of intact-cell methods for their key experiments, which is the best way to address αSyn homeostasis in our experience. Crosslinking in vitro, i.e., in lysates, as extensively done by Burré et al. here, leads to recovery of principally monomeric αSyn (as we emphasized: see Dettmer et al., 2013; Fig. 5) because of the quantitative depolymerization of physiological multimers by cell lysis, which prevents the detection of the characteristic αSyn multimer pattern that we invariably see upon intact-cell crosslinking (Dettmer et al., 2013). This finding was ignored in the experiments by Burré. Crosslinking of brain slices has also been challenging for us, in part due to the opening of cells by the slicing as well as a risk of over-crosslinking in intact outer-cell layers and under-crosslinking in the inner parts. Instead of slices, we recommend a protocol that involves fine mincing of tissues, followed by multiple, gentle centrifugal washes to pellet the brain pieces and then applying crosslinker only to the tissue pieces that are left largely intact.
The literature has described αSyn as an amphipathic protein that can bind to membranes but is also found abundantly in the cytosol, and we believe that dynamic processes—frequent association with and dissociation from membranescharacterize αSyn homeostasis in an intact, healthy cell. (Parenthetically, Burré et al. again claim [see also Burré et al., 2013] that we postulated an entirely ‘stable’ tetramer in cytosol in our first study [Bartels et al., 2011], but that is incorrect; we postulated a metastable nature of the tetramers we observed, did not use the term stable, and explicitly proposed a search for small molecules that could stabilize them therapeutically [Bartels et al., 2011].) Emerging data, including the two new studies discussed here, point at physiological multimerization being a key part of αSyn biology. Further work is now needed to fully characterize the physiological multimers with regard to their exact subcellular localizations and half-life as well as factors involved in their assembly and disassembly.
In review, we described the tetrameric and cytosolic character of αSyn multimers (Bartels et al., 2011; Dettmer et al., 2013), W. Wang et al. their potential tetrameric and dynamic character in vitro, Westphal and Chandra their tetrameric but apparently "inactive" character, and now L. Wang et al. point out their active function on synapses, while Burré et al. characterize higher-n multimers on membranes, describing the monomeric protein as an inactive cytosolic form. Further work will bring these partially disparate observations together. Given the variety of conformations and assembly states observed to date and the potential multiple physiological functions of αSyn, it seems that both structure and functional activity are context dependent for αSyn. It might thus be inappropriate to assign only one “correct” structure, since a variety of physiological conformations are likely to be in a constant equilibrium, governed by the functional state of the cell. Given the α-helical structure of some of these species, as also discussed here by Burré et al., αSyn multimers should be protective against beta-sheet rich aggregates of αSyn that characterize the synucleinopathies. We therefore conclude that it is important to study physiological multimers, as their destabilization to monomers promises to be one of the earliest events in human synucleinopathies, suggesting entirely novel strategies for treatment.
References:
Wang L, Das U, Scott DA, Tang Y, McLean PJ, Roy S. α-synuclein multimers cluster synaptic vesicles and attenuate recycling. Curr Biol. 2014 Oct 6;24(19):2319-26. Epub 2014 Sep 25 PubMed.
Bartels T, Choi JG, Selkoe DJ. α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011 Aug 14;477(7362):107-10. PubMed.
Dettmer U, Newman AJ, Luth ES, Bartels T, Selkoe D. In vivo cross-linking reveals principally oligomeric forms of α-synuclein and β-synuclein in neurons and non-neural cells. J Biol Chem. 2013 Mar 1;288(9):6371-85. Epub 2013 Jan 14 PubMed.
Westphal CH, Chandra SS. Monomeric synucleins generate membrane curvature. J Biol Chem. 2013 Jan 18;288(3):1829-40. Epub 2012 Nov 26 PubMed.
Gould N, Mor DE, Lightfoot R, Malkus K, Giasson B, Ischiropoulos H. Evidence of native α-synuclein conformers in the human brain. J Biol Chem. 2014 Mar 14;289(11):7929-34. Epub 2014 Jan 28 PubMed.
Burré J, Sharma M, Südhof TC. α-Synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation. Proc Natl Acad Sci U S A. 2014 Oct 7;111(40):E4274-83. Epub 2014 Sep 22 PubMed.
Burré J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Südhof TC. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010 Sep 24;329(5999):1663-7. PubMed.
Burré J, Vivona S, Diao J, Sharma M, Brunger AT, Südhof TC. Properties of native brain α-synuclein. Nature. 2013 Jun 13;498(7453):E4-6; discussion E6-7. PubMed.
Wang W, Perovic I, Chittuluru J, Kaganovich A, Nguyen LT, Liao J, Auclair JR, Johnson D, Landeru A, Simorellis AK, Ju S, Cookson MR, Asturias FJ, Agar JN, Webb BN, Kang C, Ringe D, Petsko GA, Pochapsky TC, Hoang QQ. A soluble α-synuclein construct forms a dynamic tetramer. Proc Natl Acad Sci U S A. 2011 Oct 25;108(43):17797-802. Epub 2011 Oct 17 PubMed.
Make a Comment
To make a comment you must login or register.