Memory Slips as Soon as Amyloid Appears, Two Decades Before Dementia
Quick Links
How early in Alzheimer’s disease does a person’s memory falter? As soon as amyloid starts to build up, according to speakers at the Alzheimer’s Association International Conference in Chicago July 22–26. Even before an amyloid scan turns officially positive, subthreshold accumulation correlates with subtle memory deficits and presages future decline. Researchers running the A4 secondary prevention trial of late-onset AD noted that cognitive deficits are already detectable at baseline in this otherwise preclinical population. Swedish researchers attempted to quantify a rate of decline at the preclinical stage, to estimate what size trials will be needed to detect drug effects at this time point.
- People accumulating amyloid below threshold levels decline on memory tests.
- In preclinical AD trials, cognitive and functional deficits are measurable at baseline.
- To detect a slowing of this decline, preclinical trials will need to be large and long.
“This is a tremendously exciting time in AD research. Our concept of Alzheimer’s has evolved, and we now think of it as a pathophysiological continuum,” said Reisa Sperling of Brigham and Women’s Hospital, Boston. She believes the ability to detect initial cognitive changes opens the door for ever earlier-stage secondary and even primary prevention studies that are now gearing up across the field.
The Swedish group also presented an updated, detailed timeline of biomarker change in sporadic AD. As predicted, amyloid builds up long before tau neurofibrillary pathology and then cognitive change. For both amyloid and tau, cerebrospinal fluid biomarkers change up to a decade before the corresponding PET signal rises. These CSF markers precede a dementia diagnosis by 30 years, while cognitive change becomes detectable 20 years before dementia. The data reinforce the idea that Alzheimer’s is a disease that occurs over decades.
Colin Masters of the University of Melbourne, Australia, noted that data on longitudinal CSF and PET changes are beginning to converge across all the major observational studies. “We are on a steep learning curve when it comes to defining the lower thresholds and their cut points,” he wrote to Alzforum. “We are now seeing the limits of sensitivity and specificity of the various PET/CSF technologies.” The ability to detect these very small changes may further refine concepts of disease, he suggested.
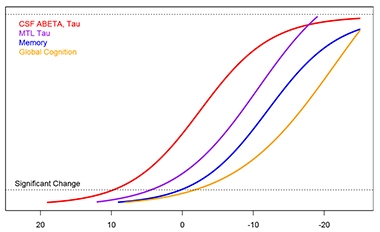
CSF and PET Separate.
Preliminary estimates from longitudinal ADNI data suggest refinements to the staging curves, with CSF markers preceding PET signal by many years. This analysis uses time to brain-wide amyloid positivity as the reference point. [Courtesy of Philip Insel and Niklas Mattsson.]
The first evidence tying subthreshold levels of brain amyloid to worse cognition came earlier this year, when William Jagust and colleagues at the University of California, Berkeley, reported that amyloid-negative ADNI participants who were accumulating plaque scored more poorly than non-accumulators on memory tests over time (May 2018 news).
In Chicago, Denise Park of the University of Texas, Dallas, corroborated this finding with data from the Dallas Lifespan Brain Study. This observational cohort study launched in 2008; every four years it examines 500 healthy and cognitively normal adults ranging from age 20 to 89, who were amyloid-negative at baseline. On average, it takes 10 or more years for plaque growth to reach the brain-wide threshold for amyloid positivity.
The news here is that cognitive effects became apparent before the positivity threshold. In the Dallas cohort, people who were accumulating subthreshold amyloid, particularly in posterior brain regions such as the precuneus and posterior cingulate, scored worse on memory tests at their first follow-up than at baseline. Executive function and other cognitive abilities remained stable (Kennedy et al., 2012; Bischof et al., 2016; Farrell et al., 2017; Farrell et al., 2018, paper in press).
Other studies have linked subjective memory complaints to elevated amyloid, even when people still score within the normal range on standard cognitive tests (Amariglio et al., 2012). The new findings show that these subjective memory complaints can be measured and tracked, Park noted. The data further strengthen the idea that any amyloid accumulation indicates a person is on the path to AD. “Amyloid foretells an individual’s future,” Park said.
Sperling reported complementary findings from A4. The trial is fully enrolled, with 1,169 participants between ages 65 and 85 being seen at 67 sites across the United States, Canada, Australia, and Japan. All participants are cognitively normal, with a CDR of zero and an MMSE score between 25 and 30. All have a positive amyloid scan, with an average SUVR of 1.33.
To find these participants, A4 researchers screened 4,486 people by amyloid PET. One-third were amyloid-positive and, importantly, compared with their amyloid-negative peers, they scored worse on the PACC, a cognitive composite designed to pick up the earliest signs of cognitive change (Jun 2014 news). The difference in scores was small, but highly statistically significant, Sperling noted. Likewise, the amyloid-positive group scored worse than amyloid-negatives on the Cognitive Function Index (CFI), a questionnaire that assesses subtle functional deficits (Mar 2015 news). As with the PACC, the difference was small but robust, with a p value of below 0.0001.
Joshua Grill of the University of California, Irvine, who studies disclosure of brain amyloid status in A4, showed data suggesting that even at baseline, people who are accumulating amyloid appear to be aware at some level that their memory has changed. Before participants learned their amyloid status, the amyloid-positive group scored slightly higher on a measure of anxiety than amyloid-negatives, and also self-reported more concerns about having Alzheimer’s.
Overall, these A4 data suggest it will be possible to detect a slowing of decline in people at preclinical stages of AD, Sperling said.
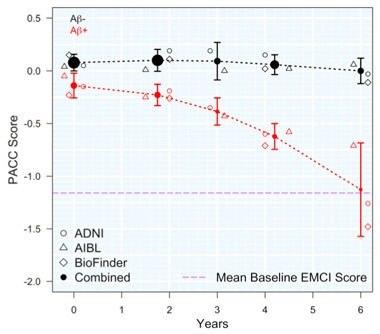
Preclinical Cognitive Decline.
Around the world, three cohorts converge to find that amyloid-positive people (red) take six years to decline from healthy control levels (black) to a marked memory deficit (purple line) on the PACC cognitive composite. [Courtesy of Oskar Hansson.]
Oskar Hansson of Lund University, Sweden, also spoke to this point. He combined six years of findings from three longitudinal cohorts—the North American ADNI, the Australian AIBL, and the Swedish Biofinder Study—to estimate the degree of memory decline in cognitively normal people with elevated brain amyloid. The three studies comprised 350 people with elevated amyloid, and 770 who were amyloid-negative. Demographic factors such as age and education varied widely between cohorts, as did study protocols, recruitment criteria, and cognitive testing.
Despite these differences, all three studies recorded a similar magnitude of decline in amyloid-positive people, amounting to about a 0.5 point deficit in PACC score over four years. Amyloid-negative participants, by contrast, remained stable. At this rate of decline, a four-year preclinical study enrolling 500 participants per arm would have 80 percent power to detect a 50 percent slowing of cognitive decline, Hansson estimated. At 800 participants per arm, a trial could detect 40 percent slowing. Large studies with longitudinal follow-up could see subtle change in cohorts that still score in the normal range on cognitive tests, Hansson concluded.
John Breitner of McGill University in Montreal challenged this conclusion, noting that a slowing of 20 to 30 percent is more realistic for most drugs. The estimates suggest that a trial in this preclinical population would need to enroll thousands of participants, Breitner said. “It looks daunting,” Breitner added, but Hansson pointed out that longer studies would have more power and could bring these numbers down. Hansson told Alzforum that a four-year study in this population would need 1,700 participants per arm to detect a 25 percent slowing of decline.
In addition, it was unclear how many of the amyloid-negative group in these three cohorts were in fact accumulating sub-threshold plaque. Because accumulators already have some cognitive decline, their presence in the control group could be lowering the power to detect an effect, noted Suzanne Hendrix of Pentara Corporation, Salt Lake City. Hansson agreed that future studies should use non-accumulators as a control.
Despite the evidence linking amyloid accumulation to cognitive decline, are plaques directly responsible for this decline? A wealth of imaging data now shows that where amyloid goes, tau tangles follow, and these correlate more closely with cognitive loss. Niklas Mattsson, also at Lund, added more data in favor of this sequence of events. Such a sequence is well-established in dominantly inherited AD, where researchers have been able to assemble a more detailed timeline of biomarker changes because they can pinpoint estimated onset age (Jul 2012 news). This has not been possible in sporadic AD preclinical cohorts, where a given person’s onset age is unknown.
To perform a similar analysis in sporadic disease, Mattsson and Philip Insel at Lund analyzed ADNI data from 43 amyloid-negative, cognitively normal controls, 34 amyloid-positive controls, 35 people with MCI due to AD, and 13 AD patients. Each participant had undergone an average of three amyloid PET scans over the course of about five years, and the researchers used these serial scans to calculate each person’s rate of amyloid accumulation. Then they applied this rate to estimate when each person would reach the threshold for amyloid positivity. For people who were already positive, the researchers regressed their accumulation rate to find the likely date when they had first become positive. The time of PET positivity was taken as year zero. This time point allowed the researchers to compare other biomarker findings and estimate when each became abnormal. Abnormality was defined as diverging more than two standard errors away from the level in amyloid-negative controls.
Using this method, Mattsson and colleagues found that CSF Aβ42 first starts dropping more than 12 years before PET scans become positive. CSF total tau starts rising around the same time, with p-tau following two years after. In other words, fluid biomarkers moved long before PET scans detected measurable protein accumulation. These data reinforce a growing realization in the field that CSF and PET measure different aspects of disease (Aug 2017 conference news). Masters noted that the relationship between tau phosphorylation and tangle formation remains murky. Some phosphorylation sites, such as Ser202/Thr205, define a pre-tangle structural change. “That structure has not yet been evaluated in relation to the various tau PET ligands. It may turn out that the CSF p-tau signal is quite different from the tau PET signal,” he said.
In the ADNI cohort, a tau PET signal in the medial temporal lobe came up about five years before brain-wide amyloid positivity. Mattsson told Alzforum that this signal was more widespread than normal age-related tau accumulation, which tends to be largely confined to the entorhinal cortex. Within a couple of years more, a tau signal became detectable in the medial parietal lobe, as well. The findings indicate that numerous biomarker changes occur before a positive amyloid PET scan flags a person as having AD. However, Mattsson noted that tau load remains low at these early stages, and does not reach the levels seen in MCI until about a decade after a positive amyloid PET scan.
Cognition changed last in this ADNI sample. Logical memory scores trended down right around the time of amyloid positivity, PACC scores followed a couple of years later, and MMSE scores waned shortly after that. Thus, cognitive change followed closely after the first signs of tau tangles spreading, in agreement with other research linking tangles to cognitive decline (Aug 2017 conference news).
Mattsson cautioned that the sample was small and these estimates are rough (see image above). More longitudinal data from larger studies will be needed to make them precise. However, the findings to date largely agree with those from the Australian Imaging, Biomarker, and Lifestyle (AIBL) Flagship Study of Ageing, which has found about a 20-year timeframe between amyloid positivity and mild cognitive impairment in sporadic disease (Dec 2014 conference news).
In sum, Mattsson’s analysis adds independent confirmation that memory decline can already be detected about 25 years before dementia onset. The data set suggests that about 35 years elapse between the first biomarker changes, at least as currently detectable, and an AD diagnosis, Mattsson said.—Madolyn Bowman Rogers
References
News Citations
- A Little Amyloid, A Lot of Trouble?
- Test Battery Picks Up Cognitive Decline in Normal Populations
- Test Tracks Preclinical Functional Decline
- Paper Alert: DIAN Biomarker Data Show Changes Decades Before AD
- CSF and Brain Markers Highlight Different Facets of Dementia
- All Signs Point to Tau Tangles as the Culprit in Fading Memory
- Large Studies Agree: Brain Amyloid Accelerates Cognitive Decline
Paper Citations
- Kennedy KM, Rodrigue KM, Devous MD Sr, Hebrank AC, Bischof GN, Park DC. Effects of beta-amyloid accumulation on neural function during encoding across the adult lifespan. Neuroimage. 2012 Aug 1;62(1):1-8. Epub 2012 May 1 PubMed.
- Bischof GN, Rodrigue KM, Kennedy KM, Devous MD Sr, Park DC. Amyloid deposition in younger adults is linked to episodic memory performance. Neurology. 2016 Dec 13;87(24):2562-2566. Epub 2016 Nov 11 PubMed.
- Farrell ME, Kennedy KM, Rodrigue KM, Wig G, Bischof GN, Rieck JR, Chen X, Festini SB, Devous MD Sr, Park DC. Association of Longitudinal Cognitive Decline With Amyloid Burden in Middle-aged and Older Adults: Evidence for a Dose-Response Relationship. JAMA Neurol. 2017 Jul 1;74(7):830-838. PubMed.
- Amariglio RE, Becker JA, Carmasin J, Wadsworth LP, Lorius N, Sullivan C, Maye JE, Gidicsin C, Pepin LC, Sperling RA, Johnson KA, Rentz DM. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia. 2012 Oct;50(12):2880-6. PubMed.
External Citations
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.