Plasticity Hums With Protein Synthesis on Both Sides of Synapse
Quick Links
Scientists know that synaptic plasticity requires local protein production from translation of mRNAs in postsynaptic dendrites. However, local synthesis had not been clearly demonstrated on the other, presynaptic side in mature axons. A new study claims that on-the-spot translation on both sides of the synapse fine-tunes the synaptic proteome, and contributes to synaptic plasticity. In the May 17 Science, Erin Schuman, Max Planck Institute for Brain Research, Frankfurt, Germany, and colleagues publish evidence of ongoing, active protein synthesis in both pre- and postsynaptic compartments of neurons from mouse brain. Different types of synaptic plasticity stimulated protein synthesis on one or both sides of the synapse, and did so in distinct ways.
- To remodel synapses and create memories, dendrites make proteins locally.
- Axons do it, too, new study claims.
- During plasticity, proteome gets remodeled on both sides of synapse.
In the study, first authors Anne-Sophie Hafner and Paul Donlin-Asp used advanced microscopy and biochemical techniques to probe the contents of presynaptic compartments. By microscopy, they could clearly make out mRNA and ribosomes in more than 75 percent of presynaptic terminals in several regions of the adult mouse brain. Metabolic labeling detected ongoing protein synthesis in one-third of excitatory and nearly half of inhibitory presynapses; more than half of postsynaptic compartments had ongoing protein synthesis. Metabolic labeling was apparent within five minutes, indicating a “surprisingly high level of ongoing protein synthesis,” the authors wrote.
That was in unstimulated neurons. When the researchers induced synaptic plasticity, protein synthesis shot up, and in interesting ways. When the investigators tickled cultured neurons with the neurotropic factor BDNF to induce plasticity, this stimulated protein synthesis in both pre- and postsynaptic compartments. Glutamate receptor stimulation, on the other hand, increased protein production only in dendritic spines, and a cannabinoid receptor agonist increased translation only in inhibitory presynapses. The results suggest that different types of plasticity induce distinct changes in the synaptic proteome, involving both sides of the synapse.
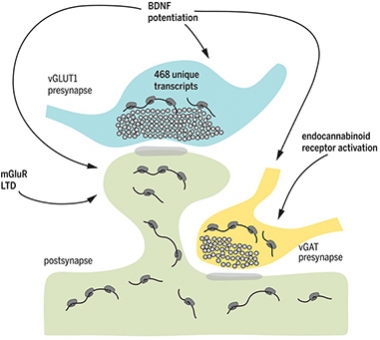
Both Sides. Plasticity induced by BDNF, glutamate receptor stimulation, or endocannabinoids stimulates local protein synthesis in pre- and postsynaptic compartments. [Courtesy of Science/AAAS.]
To probe the transcriptome of excitatory presynapses, the researchers used synaptosomes—vesicles containing synaptic contents that can be isolated and sorted according to synapse type for analysis. RNA sequencing revealed that synaptosomes harbor a diverse set of mRNAs, including some 450 transcripts enriched in excitatory synapses compared with a generic synaptosome transcriptome.
This result may raise a flag for a growing line of investigation in the neurodegeneration field, i.e., the use of nuclear RNA sequencing to characterize the transcriptome of human neurons. That type of analysis will miss information about the subcellular distribution of transcripts and local regulation of translation, Hafner told Alzforum.
How might this finding relate to Alzheimer’s disease? Robert Vassar, Northwestern University, Chicago, said presynaptic proteome remodeling could be related to memory deficits in AD. “Since axonal transport is impaired in Alzheimer’s disease, it is likely that ribosomes and messenger RNAs at the synapse will be altered, which could affect synaptic plasticity, learning, and memory,” he wrote to Alzforum.
Hafner pointed out that among the mRNAs they identified as being enriched in excitatory presynaptic boutons were mRNAs encoding eEF1A and eIF2, two regulators of protein translation. The function of these proteins seems to be impaired in people with AD (Beckelman et al., 2019; Beckelman et al., 2016). “Together, those results suggest a link between Alzheimer’s and local protein production. One would have to determine if dysregulation of protein production is a trigger of the onset of the disease or a result of it,” Hafner wrote to Alzforum.
Ulrich Hengst, Columbia University, New York, previously reported that soluble oligomeric Aβ42 alters the axonal proteome and increases axonal protein synthesis. “It will be especially interesting to determine how Aβ1-42-induced changes in axonal mRNA localization and protein synthesis affect the presynaptic proteome, and whether synaptic dysfunction in AD is at least in part mediated by changes to presynaptic protein synthesis,” he wrote to Alzforum.—Pat McCaffrey
References
Paper Citations
- Beckelman BC, Yang W, Kasica NP, Zimmermann HR, Zhou X, Keene CD, Ryazanov AG, Ma T. Genetic reduction of eEF2 kinase alleviates pathophysiology in Alzheimer's disease model mice. J Clin Invest. 2019 Feb 1;129(2):820-833. Epub 2019 Jan 22 PubMed.
- Beckelman BC, Zhou X, Keene CD, Ma T. Impaired Eukaryotic Elongation Factor 1A Expression in Alzheimer's Disease. Neurodegener Dis. 2016;16(1-2):39-43. Epub 2015 Nov 10 PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Hafner AS, Donlin-Asp PG, Leitch B, Herzog E, Schuman EM. Local protein synthesis is a ubiquitous feature of neuronal pre- and postsynaptic compartments. Science. 2019 May 17;364(6441) PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Columbia University
Axonal protein synthesis is a mechanism to alter the local proteome in a spatially and temporally restricted manner. Many studies have demonstrated its importance in virtually every aspect of axonal biology, e.g., developmental growth, regeneration, synapse formation. In contrast, the question of whether protein synthesis occurs at the mature presynapse has remained controversial.
Hafner et al. are now providing direct evidence from rodent brain and cultured neurons that protein synthesis happens routinely at both sides of mature CNS synapses. While the function of presynaptic protein synthesis remains unknown, the finding that protein synthesis is differentially regulated at excitatory and inhibitory presynapses, and in response to distinct forms of plasticity, suggests a direct role in synapse function.
Exposure to soluble oligomeric Aβ1-42 changes the axonally localized transcriptome and increases axonal protein synthesis. In the context of AD, it will be especially interesting to determine how Aβ1-42-induced changes in axonal mRNA localization and protein synthesis affect the presynaptic proteome, and whether synaptic dysfunction in AD is at least in part mediated by changes to presynaptic protein synthesis.
Keck School of Medicine
Dr. Hafner is correct in stating that analyzing the nuclear transcriptome "will miss information about the subcellular distribution of transcripts and local regulation of translation." I spearheaded a study of the synaptic transcriptome in MCI/incipient AD brains, published in 2009. Although the study focused on dendritic mRNA transcripts, we also detected axonal transcripts with altered expression.
References:
Williams C, Mehrian Shai R, Wu Y, Hsu YH, Sitzer T, Spann B, McCleary C, Mo Y, Miller CA. Transcriptome analysis of synaptoneurosomes identifies neuroplasticity genes overexpressed in incipient Alzheimer's disease. PLoS One. 2009;4(3):e4936. PubMed.
Max-Planck-Institute for Brain Research
I’d like to thank Celia Williams for bringing her paper to my attention. It is very interesting. I am not surprised she identified both dendritic and axonal genes. Synaptoneurosomes, as she beautifully shows in EM, have the particularity to be composed of both a presynaptic terminal and a postsynaptic spine, both with closed membranes.
In fact, I have compared the excitatory presynaptic transcriptome and the list of dysregulated transcripts in Alzheimer from Dr. Williams’ study. Among the 13 presynaptically localized transcripts that are dysregulated during AD, there are: KIF1A, a kinesin implicated in anterograde axonal transport; SNRK, a kinase identified as a potential mediator of neuronal apoptosis; and GRK2 (ADRBK1), a kinase already identified as an early marker of AD.
Make a Comment
To make a comment you must login or register.