Plasma NT1: This Tau Snippet Predicts Cognitive Decline in Alzheimer’s
Quick Links
For Alzheimer’s disease patients, biofluid markers that can forewarn them that their memories will soon start to slide could help them plan for the future and prompt them to join a therapeutic trial.
- Researchers quantified NT1 levels using cognitively normal participants in the Harvard Brain Aging study.
- Their NT1 levels predict cognitive decline, tau accumulation, and neurodegeneration.
- This immunoassay may offer an inexpensive blood test for early AD.
Past work has shown that a recently identified N-terminal tau fragment, measured in cerebrospinal fluid or blood, can distinguish between controls and AD dementia populations with high efficacy and sensitivity (Dec 2018 news). Now, a new study published in Nature Communications on November 27 reports that plasma NT1 levels can predict future cognitive decline, pathological tau accumulation, neurodegeneration, and an AD or mild cognitive impairment diagnosis in clinically normal elderly participants. Led by Jasmeer Chhatwal at Massachusetts General Hospital, Boston, and Reisa Sperling and Dennis Selkoe from Brigham and Women’s Hospital, Boston, the research holds out the promise of a relatively inexpensive immunoassay that is practicable for screening large numbers of people and that can work alongside other biomarkers in a rapidly growing field.
“This important study by Chhatwal and colleagues demonstrates the merits of a novel plasma biomarker, NT1, as a marker of early neurodegenerative changes along the AD trajectory,” Elisabeth Thijssen from Amsterdam UMC, The Netherlands, told Alzforum.
Unlike prior CSF tau tests, which use antibodies that recognize the mid-region of tau, NT1 uses antibodies specific to its N-terminus, specifically to fragments. These N-terminal fragments are actively secreted by neurons in response to Aβ pathology (Mar 2018 news). For Chhatwal and colleagues, the big question was whether this end piece of tau could forecast AD progression in individuals who have no symptoms yet. “Can we get a glimpse into what the future holds by looking at this blood marker?” Chhatwal asked.
To answer this question, the authors used ultrasensitive, single-molecule array, aka SIMOA, technology to measure NT1 and neurofilament light chain, or NfL, in blood plasma samples from 236 elderly participants from the Harvard Brain Aging study. Plasma measures were then compared with cognitive performance from the Preclinical Alzheimer’s Cognitive Composite, PET measurements of β-amyloid and tau, and hippocampal and total gray-matter volume.
They found that when a person had more NT1 at the beginning of the study, he or she tended to perform worse on cognitive tests at that time, and also decline more steeply between baseline and the follow-up exam four to six years later. This biomarker was particularly good at predicting declines in episodic memory. Only 9.7 percent of total HABS participants have progressed to MCI or AD this far into the study, but already, higher NT1 plasma levels appear associated with clinical progression.
The authors also measured participants’ amyloid plaque load using Pittsburgh compound B in their baseline PET scans. They observed that NT1 came with cognitive decline, but this relationship was stronger in people with a high than a low amyloid load. Additionally, they saw that NT1 begins to interact with PiB to predict cognitive decline at PiB levels below the “high PiB” threshold, suggesting that the NT1 assay may be highly sensitive. However, some participants whose NT1 levels were high had a negative amyloid scan. “While plasma NT1 is correlated strongly with amyloid PET buildup, it’s not a universal correlation,” Selkoe acknowledged.
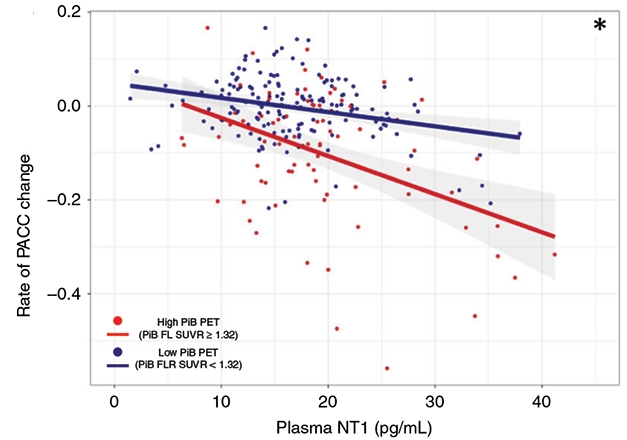
Downhill with NT1. High NT1 plasma levels correlate with cognitive decline in both high and low PiB groups, though this association was stronger in the former.
What about neurodegeneration? By tracking participants’ total gray matter and hippocampal volume for progressive atrophy, the researchers detected that NT1 was associated with shrinkage over time in both. Post hoc statistical analyses associating regional cortical thickness and NT1 hinted that high blood NT1 came with more thinning in several medial and lateral regions in people who also had high PiB. Unsurprisingly, high NT1 levels were also associated with tau accumulation as measured with Flortaucipir PET, in participants with elevated PiB burden.
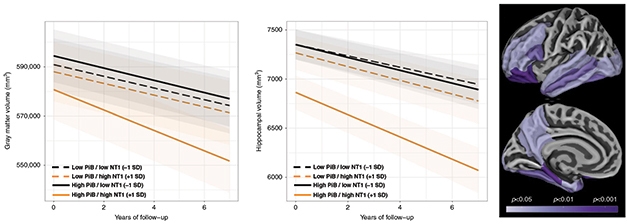
Forecasting Neurodegeneration. Higher NT1 at baseline means greater longitudinal neurodegeneration as seen by gray-matter volume (left), hippocampal volume (center), and cortical thinning (right), particularly for people with a high plaque burden (solid lines in a and b).
Finally, the researchers found that levels of plasma NfL, which has been used as a blood biomarker of axonal damage in research studies and trials, correlated with plasma NT1 levels.
Moving forward, the authors plan to compare NT1 directly to p-tau217 and p-tau181. “We don’t know the relationship of these fragments of tau at a molecular level,” said Selkoe. “For example, are p-tau-217 and NT1 the same fragment of tau? Or made by the same cleavage of tau? We have to purify NT1 from the CSF to learn about its mass spectrometry identity.”
Selkoe sees these immunoassays being used alongside one another to better detect AD decline. “We think NT1 complements the wonderful work that has been done on ptau-217 and ptau-181,” he said. “NT1 is a fellow traveler that is also abnormal in the plasma.”
NT1 antibodies are commercially available. They have not been patented, something Thijssen was excited to see. “It is good that the NT1 assay has been transferred to the SIMOA HD-1 and SP-X platforms, which are partly automated and allow for relatively easy implementation in other laboratories,” she said.—Helen Santoro
References
News Citations
Further Reading
No Available Further Reading
Primary Papers
- Chhatwal JP, Schultz AP, Dang Y, Ostaszewski B, Liu L, Yang HS, Johnson KA, Sperling RA, Selkoe DJ. Plasma N-terminal tau fragment levels predict future cognitive decline and neurodegeneration in healthy elderly individuals. Nat Commun. 2020 Nov 27;11(1):6024. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Center for Neurology and Hertie Institute for Clinical Brain Research, University of Tübingen
This is a remarkable study, clearly demonstrating the value of NT1 tau to predict cognitive decline (worsening PACC score), neurodegeneration (gray matter and hippocampal atrophy and cortical thinning), and pathological tau accumulation (FTP tau PET).
It is a nice complementary extension of prior work demonstrating that distinct fragments of tau, specifically forms of tau captured with the NT1 tau assay (with the minimal sequence requirement spanning amino acids 6–198) are specific for AD pathology (Chen et al., 2019; Mengel et al., 2020).
The findings also provide corroborative evidence to the recent study in the Swedish Biofinder cohort that NT1 tau is elevated early in disease and helps to identify individuals with future cognitive decline (Mengel et al., 2020). Interestingly, in that study we had found that NT1 tau, but not tau measured using ultrasensitive Quanterix or Roche assays, is elevated in subjects with SCD and MCI who progressed to AD dementia (but not to non-AD dementia), thereby demonstrating the specificity of NT1 tau for AD pathobiology.
Taken together, these studies provide a strong body of evidence that NT1 tau should be tested in combination with markers of phospho-tau, particularly tau T181P and T217P, to study the (temporal) relationship of these markers, and whether a combination might further improve their diagnostic and predictive usefulness.
References:
Chen Z, Mengel D, Keshavan A, Rissman RA, Billinton A, Perkinton M, Percival-Alwyn J, Schultz A, Properzi M, Johnson K, Selkoe DJ, Sperling RA, Patel P, Zetterberg H, Galasko D, Schott JM, Walsh DM. Learnings about the complexity of extracellular tau aid development of a blood-based screen for Alzheimer's disease. Alzheimers Dement. 2019 Mar;15(3):487-496. Epub 2018 Nov 9 PubMed.
Mengel D, Liu W, Glynn RJ, Selkoe DJ, Strydom A, Lai F, Rosas HD, Torres A, Patsiogiannis V, Skotko B, Walsh DM. Dynamics of plasma biomarkers in Down syndrome: the relative levels of Aβ42 decrease with age, whereas NT1 tau and NfL increase. Alzheimers Res Ther. 2020 Mar 19;12(1):27. PubMed.
Mengel D, Janelidze S, Glynn RJ, Liu W, Hansson O, Walsh DM. Plasma NT1 Tau is a Specific and Early Marker of Alzheimer's Disease. Ann Neurol. 2020 Nov;88(5):878-892. Epub 2020 Sep 25 PubMed.
Make a Comment
To make a comment you must login or register.