And Then There Were Four: A New Meningeal Membrane Discovered
Quick Links
Time to rewrite the brain anatomy textbook? In addition to the outermost dura, middle arachnoid, and innermost pia membranes surrounding the brain, researchers led by Kjeld Møllgård, University of Copenhagen, and Maiken Nedergaard, University of Rochester Medical Center, New York, identified another layer, called the subarachnoid lymphatic-like membrane. In the January 6 Science, they reported that SLYM lies between the arachnoid and pia membranes, splitting the subarachnoid space. It wraps around blood vessels traversing the pia, restricting solute movement between the blood and cerebrospinal fluid. The membrane also harbors immune cells that likely aid in immune surveillance. Their numbers swell in aged mice, and a physically damaged SLYM compromises glymphatic flow, the scientists report. Both findings might have implications for neurodegenerative disease.
“This is another exciting and thought-provoking work from the group of Maiken Nedergaard that calls for revision to our understanding of meningeal function and anatomy,” wrote Jonathan Kipnis and Leon Smyth, Washington University in St. Louis. Per Kristian Eide, University of Oslo, Norway, called this paper groundbreaking. “The transport of cerebrospinal fluid in the subarachnoid compartment seems to be far more organized than we previously thought,” he wrote (comments below).
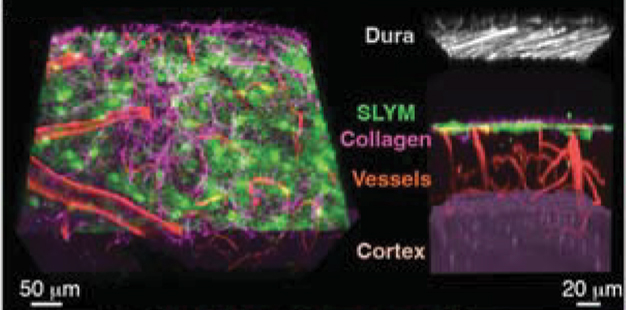
A Fourth Brain Layer. Beneath the dura mater (gray), the newly identified SLYM (green) splits the subarachnoid space into an outer compartment and a vessel-rich inner compartment (red) lining brain tissue (purple). [Courtesy of Møllgård et al., Science, 2023.]
Cerebrospinal fluid bathes the brain, bringing in nutrients and clearing out cellular junk. But how the fluid moves through the relatively wide space between the arachnoid and pia mater is unclear. Co-first authors Møllgård, Felix Beinlich, Peter Kusk, and Leo Miyakoshi, all at U Copenhagen, aimed to find out.
The researchers used in vivo two-photon microscopy to peer into the cortices of 3- to 4-month-old mice expressing green fluorescent protein in lymphatic endothelial cells. Lo and behold, under the arachnoid, Møllgård spied a continuous layer of lymphatic endothelial cells intertwined with collagen fibers—the SLYM. It split the subarachnoid space into an upper layer and a vessel-rich lower layer lining the brain (see image above).
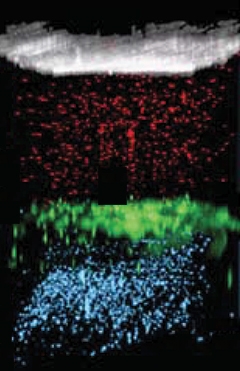
Stay in Your Lane. Fluorescently-labeled particles (red and blue) stayed on the side of the SLYM (green) where they had been injected, indicating that the SLYM is impermeable to large macromolecules. [Courtesy of Møllgård et al., Science, 2023.]
Is the SLYM permeable? The scientists injected 1 μm wide red fluorescent particles into the subdural outer layer of the subarachnoid space and blue fluorescent particles into the cisterna magna to fill the inner layer. The particles stayed where they were (see image below). So did the smaller, 3 kDa, dextran, suggesting that the SLYM prevents the exchange of most proteins, peptides, and larger molecules between the CSF and blood.
“It remains to be seen what role the SLYM has in the selective permeability of small molecular weight solutes and their glymphatic circulation,” wrote Roxana Carare, University of Southampton, U.K. (comment below).
How did the researchers know this membrane was distinct? They immunostained brain slices for various lymphatic and membrane-specific markers (see image below). The arachnoid expressed the cell adhesion proteins claudin-11 and E-cadherin. The pia was positive for the meningeal marker CRABP2 with patches of the lymphatic endothelial marker podoplanin and the lymphatic vessel marker VEGRFR3. In contrast, the SLYM widely expressed podoplanin and CRABP2 but no VEGRFR3. It also did not express the lymphatic marker LYVE, suggesting that the SLYM is indeed a meningeal layer, not a lymphatic one.

Distinct Layers. A combination of markers proved that the SLYM (green) is a unique meningeal layer that abuts blood vessels in the subarachnoid space. [Courtesy of Møllgård et al., Science, 2023.]
While all this sleuthing was done in mice, it turns out that people have this fourth membrane, too. Immunostaining human cerebral cortex slices revealed a PDPN- and CRABP2-positive membrane above the pia that ran throughout the entire subarachnoid space.
Podoplanin positivity caught the researchers’ attention because it is expressed by mesothelial membranes that encase and lubricate the movement of peripheral organs against other organs and bones. The authors think that the SLYM may act similarly in the brain, easing friction between the brain and skull and, perhaps, coating blood vessels in the subarachnoid space.
Mesothelial membranes act as an immune barrier, and so might SLYM (Mutsaers et al., 2020). In vivo imaging and immunostaining showed many white blood cells and macrophages embedded within this membrane (see image below). The leukocytes quadrupled in 12-month-old mice compared to healthy 3- to 4-month-old mice. The latter, when injected with lipopolysaccharide, an inflammatory stimulus, similarly ramped up these SLYM immune cells.
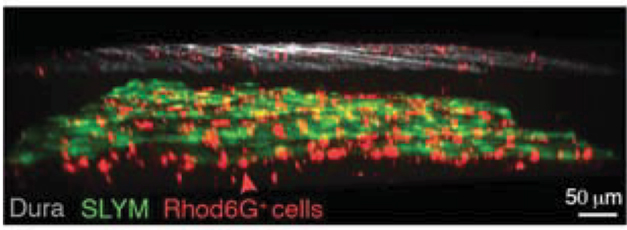
Speckled with Immune Cells. Like the dura (gray), the SLYM (green) was filled with white blood cells (red). [Courtesy of Møllgård et al., Science, 2023.]
Such an immune response has direct implications for neurodegenerative disease. A dysfunctional SLYM might blunt immune cell recruitment, the authors contend. Similarly, in mice with a damaged dura, the fluorescent dextran molecule leaked into both sides of the SLYM, suggesting that damage to the meningeal layers may also compromise the blood-brain barrier and alter glymphatic flow.
All told, the authors concluded that the SLYM is a new meningeal membrane that divides the subarachnoid space to organize CSF flow and facilitate immune surveillance.—Chelsea Weidman Burke
References
Paper Citations
- Mutsaers SE, Pixley FJ, Prêle CM, Hoyne GF. Mesothelial cells regulate immune responses in health and disease: role for immunotherapy in malignant mesothelioma. Curr Opin Immunol. 2020 Jun;64:88-109. Epub 2020 May 30 PubMed.
Further Reading
Primary Papers
- Møllgård K, Beinlich FR, Kusk P, Miyakoshi LM, Delle C, Plá V, Hauglund NL, Esmail T, Rasmussen MK, Gomolka RS, Mori Y, Nedergaard M. A mesothelium divides the subarachnoid space into functional compartments. Science. 2023 Jan 6;379(6627):84-88. Epub 2023 Jan 5 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Washington University in St. Louis, School of Medicine
Washington University in St. Louis
This is another exciting and thought-provoking work from the group of Maiken Nedergaard that calls for revision to our understanding of meningeal function and anatomy.
The authors propose the existence of a previously undiscovered fourth meningeal layer, nicknamed SLYM. This layer of mesothelium, which can be marked by the expression of Prox1, appears to functionally subdivide the fluid spaces of the brain into separate compartments. They go on to show that this SLYM layer is important to CNS waste clearance and is a hub of immune interaction during inflammation. It will be exciting to further understand the nature of these cells, and how they contribute to disease. Interestingly, this work comes out the week when a decline in “disruptive science” is being discussed (Kozlov, 2023). In the field of neuroimmunology/brain borders there is no shortage of “disruptive” works!
References:
Kozlov M. 'Disruptive' science has declined - and no one knows why. Nature. 2023 Jan 4; PubMed.
Oslo University Hospital / University of Oslo
Up to now, it has been thought that the brain is covered by three meningeal layers—the pia, the arachnoid, and the dura mater nearby the inner skull. In this groundbreaking Science paper from the Nedergaard group, they provide evidence for a fourth meningeal layer, namely a subarachnoid lymphatic-like membrane that they denote SLYM. SLYM shows similarities with the mesothelium that covers other body organs and serves multiple functions, such as mechanical—preventing tissue friction during motion—and immunological, forming an immune barrier.
SLYM compartmentalizes the subarachnoid space by its proximity to the blood vessels residing within this compartment. They further provide evidence that the SLYM serves as a barrier for larger substances above 3 kDa in size, and between the outer and inner compartments of the subarachnoid space. This provides an anatomical basis for directional transport of fluids and substances of larger size, be they metabolites, immune products, etc., between the brain and the meningeal lymphatic structures. Thereby, the transport of cerebrospinal fluid in the subarachnoid compartment seems to be far more organized than we previously thought. Another important aspect of this work is that the SLYM subdividing the subarachnoid compartment harbors immune cells and changes during inflammation or with increasing age.
For sure, this highly significant work will have a wide impact and spark renewed interest, for example concerning the complex role of the subarachnoid space for immune surveillance, and directional subarachnoid transport of cerebrospinal fluid to and from perivascular spaces of the brain and to and from lymphatic vessels and veins of dura. From a clinical perspective, the discovery of a fourth meningeal layer may have implications for a wide range of brain diseases such as neurodegenerative disorder and dementias, stroke, post-traumatic brain injury, and neuro-immunological disorders.
University of Southampton School of Medicine
We knew that leptomeningeal cells associated with the arachnoid show variable immunocytochemical and ultrastructural characteristics depending on their location or the structures that they associate with (for a review see Weller et al., 2018). For example, we knew there are tight junctions on the superficial layer of arachnoid where it abuts the dura mater. This work beautifully shows that this superficial layer forms a distinct layer that the authors named SLYM, that it has different immunocytochemical markers compared to the rest of the arachnoid, and that it also abuts the venous wall, appearing to function much like arachnoid villi in humans.
It remains to be seen what role SLYM has in the selective permeability of small molecular weight solutes and their glymphatic circulation. As it is in the outer arachnoid layer, SLYM is unlikely to be of much relevance to intramural periarterial drainage that occurs along basement membranes of capillaries and arteries where Aβ deposits in cerebral amyloid angiopathy.
References:
Weller RO, Sharp MM, Christodoulides M, Carare RO, Møllgård K. The meninges as barriers and facilitators for the movement of fluid, cells and pathogens related to the rodent and human CNS. Acta Neuropathol. 2018 Mar;135(3):363-385. Epub 2018 Jan 24 PubMed.
Weill College Medicine, New York
This paper describes a fourth meningeal layer (SLYM) located between the arachnoid mater and the subarachnoid space, effectively partitioning the subarachnoid space into two distinct compartments. Earlier studies in a variety of species had revealed that the subarachnoid space is partitioned by complex membranous structures suggesting diverse paths for the circulation of the cerebrospinal fluid (CSF) (e.g., Woollam et al., 1955; Nabeshima et al., 1975; Schachenmayr et al., 1978; Zhang et al., 1990; Weller et al., 2018). The present paper provides novel anatomical, functional, and molecular evidence supporting a continuous mesothelial-like membrane delimiting two non-communicating sub-arachnoid compartments.
The implications for these findings are significant. Considering the diverse CSF/interstitial fluid (ISF) clearance pathways that have been proposed (perivascular, glymphatic, intramural periarterial, CSF/interstitial fluid mixing), the observation that the subarachnoid space is partitioned in separate compartments provides the anatomical bases for multiple clearance pathways, perhaps operating simultaneously. Furthermore, the SLYM’s villous expansions in the venous sinuses shed light on the mechanisms of CSF reabsorption in rodents, a species lacking arachnoid granulations. The existence of a "sleek” mesothelial membrane enveloping the brain suggests a dampening system protecting the brain from mechanical stresses during head movements. If so, stiffening of this membrane by disease or aging could render the brain more susceptible to microtraumas.
The presence of immune cells embedded in the SLYM provides further insight into the intricacy of the immune surveillance of the brain. Since these cells have the potential to produce damaging cytokines and reactive oxygen species, neuroinflammation may lead to damage to the barriers separating the subarachnoid compartments, as well as the arachnoid barrier, altering CSF clearance and leading to accumulation of unwanted metabolites and proteins. The implications of such disruption for neuroinflammatory, neurovascular, and neurodegenerative diseases remain to be explored, and may lead to new insights into disease mechanisms.
This study exemplifies how applying cutting-edge technologies sheds new light on structures, such as the brain’s coverings, which have been investigated for centuries.
References:
WOOLLAM DH, MILLEN JW. The perivascular spaces of the mammalian central nervous system and their relation to the perineuronal and subarachnoid spaces. J Anat. 1955 Apr;89(2):193-200. PubMed.
Nabeshima S, Reese TS, Landis DM, Brightman MW. Junctions in the meninges and marginal glia. J Comp Neurol. 1975 Nov 15;164(2):127-69. PubMed.
Schachenmayr W, Friede RL. The origin of subdural neomembranes. I. Fine structure of the dura-arachnoid interface in man. Am J Pathol. 1978 Jul;92(1):53-68. PubMed.
Zhang ET, Inman CB, Weller RO. Interrelationships of the pia mater and the perivascular (Virchow-Robin) spaces in the human cerebrum. J Anat. 1990 Jun;170:111-23. PubMed.
Weller RO, Sharp MM, Christodoulides M, Carare RO, Møllgård K. The meninges as barriers and facilitators for the movement of fluid, cells and pathogens related to the rodent and human CNS. Acta Neuropathol. 2018 Mar;135(3):363-385. Epub 2018 Jan 24 PubMed.
University of Bern
Uppsala University and Karolinska Institute
University of Bern
University of Helsinki and Wihuri Research Institute
University of California San Francisco
This report from the Nedergaard group claims to have discovered a fourth layer of the meninges that until now has been overlooked. The authors claim that this membrane, called “subarachnoid lymphatic-like membrane” (SLYM), acts as a barrier that functionally divides the subarachnoid space into two separate compartments—an outer and an inner one. If true, this discovery would be groundbreaking and change our perceptions of the meningeal barrier, how cerebrospinal fluid circulates, and how immune cells gain access or leave the central nervous system. However, unfortunately, we respectfully submit that the claims are invalid.
The authors, reviewers, and most Alzforum commentators seem to have accepted the authors’ interpretations on face value, without critical consideration of the evidence presented and what is already known in the literature. An example is Figure 2A, which is interpreted as evidence of the barrier function of the fourth layer, even though the arachnoid barrier layer is not visible. The axiom “absence of evidence is not evidence of absence” is ignored.
With the goal of finding the truth for us, scientists and clinicians, as well as, foremost, patients with neurodegenerative disease and their families, we critically assessed the evidence presented in Møllgård et al., 2023, and in the relevant literature. We found that the authors’ interpretations presented in this paper are flawed and have ignored centuries of work by prior investigators.
Point 1: No convincing evidence is presented for the existence of a fourth membrane of the meninges separating two compartments of the subarachnoid space. Although the finding of a Prox1-positive monolayer of meningeal cells, which the authors designate the “SLYM membrane,” is novel, it is not a separate layer but is, in fact, the well-documented innermost layer of the arachnoid mater. This inner layer of arachnoid is adjacent to, but morphologically distinct from, the outer layer of arachnoid barrier cells. Electron microscopic imaging in multiple laboratories, not cited by Møllgård et al., 2023, has clearly demonstrated the existence of multiple layers of arachnoid cells in all mammals investigated, including the mouse, beginning with the landmark work from the laboratories of Tom Reese and Milton Brightman at NIH (Nabeshima et al., 1975).
The multilayered nature of the arachnoid was confirmed by Møllgård et al., 2023, through images showing the Prox1-GFP+ cellular layer directly adjacent to the outer layer of E-cadherin-positive arachnoid barrier cells (Figures 4E and S6), with no space between the two cell layers. This means the “outer subarachnoid space” depicted in Figure 2A is in fact the subdural space. This space is normally not present but forms after trauma or mechanical forces that result in subdural hematoma (Haines, 1991).
In the case shown in Figure 2A, mechanical separation was caused by injection under the dura mater of 100-200 microliters of fluid and microspheres, as described in the paper’s supplementary Methods section. Considering that the entire CSF volume in an adult mouse is only 30 to 35 microliters, 100-200 microliters is unphysiologically large. The precise size of the subdural space caused by the injection is unclear. As Figure 1A is a composite of two separate two-photon imaging scans, one before and the other after removal of the dura, the size of the “space” between “SLYM” and dura seems to be the authors’ estimate, whereas in Figure 2A the separation is 150-200 µm. Regardless of their size, these large separations are in sharp contrast to the normally close apposition of the arachnoid and dura.
Point 2: No convincing evidence is presented for the Prox1-positive layer of cells acting as a barrier between two compartments. Images of tracers in Figure 2A do not show the location of the arachnoid barrier layer. Based on the Prox1/E-cadherin data, the arachnoid barrier layer is immediately adjacent to the Prox1-positive “SLYM” layer. The close apposition of “SLYM” and the arachnoid barrier layer invalidates interpretations of the barrier properties of “SLYM.” Instead, Figure 2A reproduces previous work documenting the barrier function of the leptomeninges at the level of the arachnoid barrier layer (Krisch and Oksche, 1983; Balin et al., 1986).
Point 3: No convincing evidence is presented to justify the description of "SLYM” as “lymphatic-like” or “mesothelium." These terms appear to be based on the expression of Prox1, an important gene for lymphatic vessel development, and podoplanin, which is expressed by mesothelial cells lining the peritoneum and other body cavities. However, using these terms for a layer of the arachnoid is misleading because neither Prox1 nor podoplanin are unique to lymphatic endothelial cells or mesothelial cells. Prox1 is expressed by many neurons, cells of the eye lens, oligodendrocytes, satellite cells in skeletal muscle and multiple other cells not considered “lymphatic-like” or “mesothelium” (Oliver et al., 1993; Kivelä et al., 2016). Podoplanin is broadly expressed in fibroblasts throughout the body of mice (Muhl et al., 2020), and was reported in this paper to be found not only in “SLYM” but also in cells of arachnoid trabeculae and some regions of pia (Figures 3B, 3C, and 3E).
Point 4: No convincing evidence is presented to justify the proposed functions of “SLYM” as a (i) frictionless membrane during brain motion; (ii) mouse equivalent of human arachnoid villi or granulations; (iii) route for CSF outflow; or (iv) scaffold for immune cell adherence or trafficking to or from the subarachnoid space. Although the first three claimed functions are purely speculative, the fourth is a feature of the arachnoid mater documented by dozens, if not hundreds, of studies showing immune cells residing in, or trafficking through, the subarachnoid space bordered by the arachnoid above and pia below (Engelhardt et al., 2017).
It is unfortunate that the peer review process of such a prestigious journal as Science failed in this case; even so, we hope that the search for the truth will lead to a course of action that corrects invalid interpretations and misleading citation of the relevant literature.
References:
Nabeshima S, Reese TS, Landis DM, Brightman MW. Junctions in the meninges and marginal glia. J Comp Neurol. 1975 Nov 15;164(2):127-69. PubMed.
Haines DE. On the question of a subdural space. Anat Rec. 1991 May;230(1):3-21. PubMed.
Krisch B, Leonhardt H, Oksche A. The meningeal compartments of the median eminence and the cortex. A comparative analysis in the rat. Cell Tissue Res. 1983;228(3):597-640. PubMed.
Balin BJ, Broadwell RD, Salcman M, el-Kalliny M. Avenues for entry of peripherally administered protein to the central nervous system in mouse, rat, and squirrel monkey. J Comp Neurol. 1986 Sep 8;251(2):260-80. PubMed.
Oliver G, Sosa-Pineda B, Geisendorf S, Spana EP, Doe CQ, Gruss P. Prox 1, a prospero-related homeobox gene expressed during mouse development. Mech Dev. 1993 Nov;44(1):3-16. PubMed.
Kivelä R, Salmela I, Nguyen YH, Petrova TV, Koistinen HA, Wiener Z, Alitalo K. The transcription factor Prox1 is essential for satellite cell differentiation and muscle fibre-type regulation. Nat Commun. 2016 Oct 12;7:13124. PubMed.
Muhl L, Genové G, Leptidis S, Liu J, He L, Mocci G, Sun Y, Gustafsson S, Buyandelger B, Chivukula IV, Segerstolpe Å, Raschperger E, Hansson EM, Björkegren JL, Peng XR, Vanlandewijck M, Lendahl U, Betsholtz C. Single-cell analysis uncovers fibroblast heterogeneity and criteria for fibroblast and mural cell identification and discrimination. Nat Commun. 2020 Aug 7;11(1):3953. PubMed.
Engelhardt B, Vajkoczy P, Weller RO. The movers and shapers in immune privilege of the CNS. Nat Immunol. 2017 Feb;18(2):123-131. Epub 2017 Jan 16 PubMed.
Department of Pathology, RUSH Medical College
The anatomy and function of the meninges and perisinus region are incompletely characterized, but highly relevant to brain physiology and diseases. This work, along with other recent work in the field, shows that the anatomy and function of the meningeal layers is far more intricate and intriguing than we had all realized.
The findings are consistent with new data that also show arachnoid granulations in humans are lymphatic gateways replete with immune cells and are sites of antigen presentation.
It has been suspected for years that mice also have rudimentary arachnoid granulations. Further characterization of this meningeal tissue in mice will clarify the similarities and differences, and appropriateness of models used to study specific factors in human disease.
University of Tokyo
The University of Tokyo
The exciting study by the Nedergaard group discovered a previously unrecognized meningeal layer, called SLYM, that is distinct from other traditional meningeal layers. SLYM subdivides the subarachnoid space into two compartments and limits exchange of molecules, suggesting that cerebrospinal fluid circulation is organized in more complicated ways than previously acknowledged.
In addition to its role in CNS immunity, it will be very interesting to see how SLYM regulates CSF waste clearance, and how it ultimately impacts various brain diseases including neurodegenerative disorders.
Rockefeller Neuroscience Institute of West Virginia University
A major strength of this study is the functional data showing fluid and tracer movement in the subarachnoid space of rodents. Physiological studies and characterization using in vivo imaging in adult humans may uncover novel neurotherapeutics as well as new techniques of drug delivery. Understanding more about the subarachnoid space compartments and how small molecules interact and are dysregulated within them in aging and disease will be important.
It is clear that perivenous architectures and function are not fully understood across species. Filling these knowledge gaps is a critical area of neuroscience and of direct relevance to diseases such as Alzheimer’s and neurodegeneration.
University of Rochester Medical Center
University of Copenhagen
University of Copenhagen
We thank our colleagues for their collegial comments regarding our study describing a fourth meningeal layer, which we have named the SLYM layer (Møllgård et al., 2023). Our colleagues raise three sets of critique: They state that SLYM is identical to the arachnoid membrane, that the data does not show that SLYM has a barrier function and that the subarachnoid space therefore not is compartmentalized. They also state that no convincing evidence is presented to justify the description of “SLYM” as “lymphatic-like” or as a “mesothelium.”
We believe that it is important to note that most of the critique is based on old histological studies of postmortem tissue. The original studies of meningeal membranes surrounding the adult brain date from the 1920s through the mid ’80s and are based on ordinary histology and EM analysis. The fixation techniques used in these classical histological methods yield significant changes in tissue morphology, in particular affecting fluid-filled spaces; fixatives are hyperosmotic, resulting in deformation and shrinkage of the thin meningeal membranes, and their artifactual adhesion to adjacent layers. This typically leads to the collapse and often disappearance of smaller fluid-filled spaces, a limitation of classical histology that has been well-recognized for decades. The more recent phenotypic characterization of the meningeal layers has primarily been done in the developing brain, which may not be representative of adult meningeal phenotype.
No convincing evidence is presented for the existence of a fourth membrane of the meninges separating two compartments of the subarachnoid space.
Our study is based on a combination of ex vivo immunophenotyping and in vivo functional studies. To our knowledge, the barrier functions of the meningeal layers had never before been studied using real-time 2-photon imaging, which is free of fixation artifacts. Our report defines a subdivision of the subarachnoid space into spatially and functionally distinct compartments, separated by a Prox1-defined lymphatic mesothelium. We should note that our critics have little experience with, and neglect the importance of, optical imaging in live mice. This was a critical part of our study, and the means by which we identified the subcompartmentalization of the subarachnoid space.
Our study was designed to understand how fluid flow is organized in the large subarachnoid compartment. To achieve this end, we needed to collect in vivo data, since it is known that fluid-filled compartments are deformed and essentially disappear during tissue fixation. (Please see our prior publication: Mestre et al., 2018. The movie included in this publication, below, shows that the CSF-filled perivascular spaces do not survive fixation.) The same phenomenon, cavity disappearance revealed only by their surviving boundary membranes and cells in ex vivo histology, was noted with the recent designation of a new organ, the interstitium (Benias et al., 2018; see also Scientific American article, 2018). This study revealed that the collagen surrounding peripheral organs does not form the dense wall observed in histological sections, but rather that the connective tissue surrounding organs is in fact an “open, fluid-filled highway,” whose internal structure and porosity are lost with fixation. This discovery shares many similarities with our report on SLYM. In essence, the collapse of the subarachnoid space in histological sections is the reason that SLYM appeared to merge with other meningeal layers, such that its functional significance in separating the subarachnoid space into discrete compartments was not previously described, and in fact could not have been done before the advent of the live in vivo analysis that we conducted.
Our colleagues refer to a study by Nabeshima et al., 1975, of the spinal and cerebral meninges using EM and freeze-fracture. It is important to notice that the spinal meninges differ significantly from the brain meninges, and that the single-layered, thin mesothelium will be destroyed during freeze-fracture procedure. We would like to refer our colleagues to several newer studies that are more comparable to our study, including studies by Krisch, Leonhardt and Oksche, 1983, 1984). in which the meninges surrounding the mouse cortex were studied by tracer injection. Fig. 1 shows a schematic drawing from these authors illustrating the meningeal layers. Using their original nomenclature and moving down from the skull to the brain, the meningeal layers were defined here as: (1) dura, (2) the inner dura layer, (3) the neurothelium and (4) the outer arachnoid layer. Below the outer arachnoid layer is the (5) cerebrospinal fluid (CSF)-filled arachnoid space, followed by (6) the inner arachnoid layer, and (7) the outer pial layer. The inner arachnoid layer and the outer pial layer are often fused, and the resultant dual layer is called the intermediate lamella. Below the intermediate lamella is (8) the pial space, the floor of which is created (9) by the inner pial layer. (10) A subpial space is also described. Injection of a tracer, horseradish peroxidase (HRP) in either the arachnoid or pial spaces demonstrated that the intermediate lamella is indeed a barrier that separates the CSF-filled arachnoid and pial compartments.
Fig. 1. The subarachnoid compartment is subdivided into two functional compartments in vivo. The composite panel replicates figures from Krisch, Leonhardt, and Oksche (1983) schematic diagrams. The top diagram depicts the meningeal layers covering cortex (Figure 30). The middle diagram (Figure 8) shows HRP injection in the arachnoid space (A). Note that the dual-layered intermediate lamella consisting of the inner arachnoid layer (top) and the outer pial layer (lower) creates a barrier that prevents that HRP entering the pial space. The lower diagram (Figure 6) shows HRP injection in the pial space. The authors conclude that intermediate lamella again acts as a barrier that prevents HRP from entering the arachnoid space.
The barrier function of this intermediate lamella was defined by injecting horseradish peroxidase (HRP) into either the arachnoid or the pial spaces (Krisch et al., 1983, 1984). Fig. 2 displays the original data by which the authors demonstrated the existence of two separate CSF-filled compartments: the arachnoid space (A), and the pial space (P). The barrier between the two compartments is created by the inner arachnoid layer and the outer pial layer that together create the intermediate lamella that effectively separates the subarachnoid space into two distinct compartments.
Krisch et al., 1983, concludes: “Due to the development of the pia mater and the arachnoidea from a common matrix primitiva and due to the cytologic characteristics common to the pia mater and the arachnoidea, one should avoid the terms ‘pia’ and ‘arachnoidea.’ Both terms, having their origin in gross anatomy, should be replaced by the terms of ‘inner, intermediate,’ and ‘outer leptomeninges’ encompassing the inner and outer leptomeningeal spaces” (Krisch et al., 1983). These observations are strikingly similar to what we reported in Møllgård et al., 2023.
Fig. 2. The arachnoid and pial CSF-filled spaces are separated by the intermediate lamella (IL). This composite figure is taken from Krisch, Leonhardt, and Oksche (1983) schematic diagrams illustrating their findings. A, HRP was injected in the arachnoid space (A, red arrows) (Figure 7), or B, in the pial space (P, blue arrows) surrounding cortex (Figure 9). The HRP injections demonstrate that the intermediate lamella consisting of the inner arachnoid and outer pial layers creates a barrier that subdivides the subarachnoid space into two distinct compartment similar to Møllgård et al., 2023 (Figure 2).
No convincing evidence is presented for the Prox1-positive layer of cells acting as a barrier between two compartments.
Our critics state that we injected in the subdural space an artificial space created when dura and the outer arachnoid layer (EA) are separated by cleavage of the inner dura layer (Fig. 1). It is important here to note that the outer arachnoid layer (EA) is a barrier expressing the tight junction claudin-11 (Brøchner, et al., 2015), and that the inner dural cells and the neurothelium (also called the dural barrier cell layer) do not express tight junctions, as such, they do not form a barrier layer facing the collagen-rich dura mater.
Fig. 3. Horseradish Peroxidase (HRP) and blood infused subdurally enters dura and accumulates along the dural collagen fibers. The figure is from J. R. Orlin, K. K. Osen, T. Hovig (1991). Note, it is clear that the HRP containing blood enter the dura and locate along the dural collagen fibers since the injection is on top of the outer arachnoid layer.
We, therefore, studied the classical literature and found that bleeding into the subdural space penetrates directly into the dura mater and distributes along the dural collagen fibers. See Fig. 3, a diaminobenzidine (DAB)-reacted Nissl-stained cryosection(s) of a pig with an experimental subdural hemorrhage, established via subdural injection of blood containing Horseradish Peroxidase (HRP). The underlying cortex is also shown (Orlin et al., 1991). The HRP reaction (black) is clearly restricted by the arachnoid barrier cell layer (ABCL); no blood enters the subarachnoid space (SAS). The key observation here is that HRP enters dura outlining the collagen fibers demonstrating that no barrier exists between the arachnoid barrier cell layer (ABCL) and the dura (Orlin et al., 1991). Similarly, Nabeshima et al. (1972) found that HRP injected into the dura reaches the arachnoid barrier cell layer. All these studies simply reflect the overwhelmingly typical clinical observation that neither acute nor subacute subdural hemorrhages traverse into the subarachnoid space in the absence of traumatic penetrating rupture of the latter.
Based on these findings—that no barrier exists between the subdural space and dura mater—we re-examined the experiments in which microspheres were injected in the upper subarachnoid space—or on top of SLYM (Møllgård et al., 2023, Figs. 2A-B). As described, the dura mater was gently punctured at the edge of the craniotomy and lifted using fine forceps, inserting a 35G needle mounted to a stereotaxic arm at an 85° angle into the subarachnoid space. Because the dural opening was larger than the needle, only a fraction of the injected solution containing microsphere solution remained in the upper subarachnoid space. The purpose of this slow infusion was to gently allow a fraction of microspheres to enter the CSF space before mounting a coverslip. Did we inject in the subdural space? We do not believe so. We are in Fig. 4A displaying a representative set of images from an experiment repeated successfully in seven mice. Second-harmonic 2-photon imaging was used to visualize the collagen fibers along with the 1 µm microspheres (red). As noted above, we would expect that microspheres injected into the subdural space on top of the outer arachnoid layer (also called the arachnoid barrier cell layer, ABCL) have free access to dura, since the inner dura layer is not a barrier. It is in panel A clear that the microspheres distributed independently of the collagen fibers. In Fig. 4B, we believe that the microspheres accidentally were injected on top of ABCL because the microspheres distributed in a pattern closely resembling that of dural collagen fibers, suggesting that the microspheres travelled in between the fibers. The similarity in distribution of collagen and microspheres were noted in two mice that were consequently excluded from the analysis shown in Fig2A of Møllgård et al., 2023.
Fig. 4. Microspheres are injected below the upper arachnoid layer. A, A representative example of one of seven successful injections in the arachnoid space. The distribution of the microspheres (red) is independent of the collagen fibers in dura (white). B, An example of microspheres distributing along the collagen fibers suggesting that this was a subdural injection on top of the outer arachnoid layer (EA). A similar pattern of microsphere distribution was noted in one additional mouse and they were both excluded from the analysis. Space bar, 50 µm.
The second line of data was generated from Z-stack reconstructed from 2-photon imaging of Prox1-EGFP reporter mice in which a very small tracer, 3 kDa, was infused into the inner subarachnoid space by cisterna magna injection. Thus, the semi-invasive procedure was > 2 cm from the field of view. In these experiments, nothing was injected into the upper subarachnoid compartment and dura was left intact. Second harmonic and regular 2-photon excitation was used to collect optical Z-stacks and cross-sections that are displayed below (Fig. 5). The images clearly demonstrate that the dura collagen fibers and SLYM are separated in some regions, but that the two layers are in close contact to each other in other areas. The remarkable observation is that the 3 kDa tracer did not leak into the upper subarachnoid space, i.e., the space between the collagen fibers and SLYM within the observation period (one to two hours). These data show that SLYM indeed acts as a barrier.
Fig. 5. Dural collagen fibers and SLYM are separated by the outer subarachnoid space. Six examples of in vivo Z-stack 2-photon imaging showing that the collagen fibers of dura (white) are separated from SLYM (green) but that the two layers can also be in close contact in other areas. The estimated distances in vivo are listed in the images. Space bar, 50 µm.
We described in the submitted manuscript the procedures in detail because the methodology was developed to interrogate the functional properties of SLYM in live brains free of fixation artifacts. Both Figs. 4 and 5 demonstrate that SLYM creates the pial space or an inner subarachnoid space CSF-filled compartment.
The major point of these two sets of in vivo studies in live mice was to show that the subarachnoid compartment is subdivided into two functional compartments. As such, our data demonstrate that subarachnoid CSF transport is organized separately within at least two subcompartments and is not simply distributed uniformly within a single large compartment, a concept that might seem simple, but substantially extends our conception beyond that provided by the current literature.
No convincing evidence is presented to justify the description of "SLYM” as “lymphatic-like” or “mesothelium," or to justify the proposed functions of “SLYM”
Our phenotypic characterization of the Prox1-EGFP+ SLYM showed that its cells can be defined as PDPN+, LYVE1−, CRABP2+, VEGFR3−, CLDN-11−, and E-Cad−. This pattern of immunostaining differs from all other layers of the meningeal layers, as documented in Figs. 3, 4, 5 and Supplementary Figs. 2-6 of Møllgård et al., 2023. We show in higher magnification some of the data displayed in Møllgård et al., 2023 (Fig. 6). A double-layered membrane consisting of two phenotypically distinct layers is clearly identified. Critique has been raised about SLYM being identical to the arachnoid barrier cell layer (ABCL). We do not think it is fruitful to go into a discussion about what is reported with regard to immunolabelling of SLYM compared to the previous published data. Our colleagues are citing developmental papers or referring to studies of peripheral tisues. It is well-known that lymphatic and mesodermal markers exhibit variations in expression pattern across species and change dramatically during development. Single-cell transcriptome data will be needed to obtain a better understanding of the meningeal layers and their evolutionary and developmental similarities and differences.
Fig. 6. SLYM express Prox1-egfp but not e-Cadherin in adult mice. A, Immunostaining of serial sections showing that the arachnoid barrier cell layer (ABCL, short arrows) is negative for Prox1-egfp, while SLYM (long arrows) is positive for Prox1-egfp (left panel). B, ABCL layer is positive for eCadherin (short arrows), while SLYM is negative (long arrows) (right panel). The pial layer is negative for both Prox1-egfp and eCadherin (arrowheads, both panels). C, immunofluoresence showing that the E-Cadherin (red) and Prox1-egfp (green) signals do not overlap but stain separate membranes, i.e., the ABCL and the SLYM layers. The panels are high magnification inserts from Møllgård et al., 2023 (Fig. 3).
Critique has also been raised about what justifies the name SLYM, i.e., Subarachnoid lymphatic-like membrane. The SLYM expresses two markers of lymphatic tissues, Prox1 and podoplanin. To be clear, the SLYM is not lymphatic tissue; it does not express VEGFR3 or LYVE1. In general, multiple lymphatic markers are needed to characterize traditional lymphatic tissue. We named the layer SLYM because its cells express the lymphatic master transcription factor, Prox1. Finally, critique is raised for the concept that SLYM may represent a brain mesothelium, analogous to that which typically surrounds other organs and body cavities. Mesothelial cells express glycoproteins that bind water to reduce friction when organs are moving. Typically, mesothelial membranes support and wrap around vessels and nerves, and are hosts for immune cells. Our conception that the SLYM comprises part of the brain’s mesothelium was based on (1) that SLYM surrounds the brain similar to the mesothelium around organs and body cavities, (2) the shared immunogenicity of SLYM with the mesothelium surrounding peripheral organs, (3) that SLYM has the classical appearance of a mesothelium consisting of a simple single-layered, squamous cell layer (Fig. 7), (4) the presence of a large number of immune cells residing in SLYM similar to mesothelium surrounding other organs, (5) that SLYM clearly surrounds the pial vasculature. Future single-cell transcriptomic data will elucidate how many similarities SLYM shares with traditional mesothelium membranes. There will be, without doubt, differences, since the mesothelium surrounding different organs differ, but the five sets of evidence listed above justify that SLYM can be described as a mesothelium—in fact, our paper was not the first to do so. Many classical histology texts refer to a layer of the meninges as mesothelia, and as far back as 1938. Weed stated that “… the villi are essentially continuations of the arachnoid mesh into the lateral walls of the great dural sinuses, so that the arachnoid mesothelium comes to lie directly beneath the vascular endothelium” (Weed, 1938; Andres, 1967; Millen and Woollam, 1962). Kirsch et al. (1983) used the term mesothelium. We suspect that our colleagues were unaware of the literature, which in fact previously demonstrated a compartmentalization of the subarachnoid space and discuss the existence of a mesothelium in the subarachnoid space.
Fig. 7. SLYM is positive for the lymphatic transcription factor PROX1. A, sagittal sections through the base of the brain stem turned upside-down to enable comparison with previous figures. The basilar artery is seen between pons (below) and SLYM above. Sections were prepared from a Prox1-egfp reporter mouse. A, Immunolabeling against egfp. Note the mesothelium appearance of the single squamous cell layer in which EGFP outline the cytosol. B, Immunolabeling of a neighboring section against Prox1. Note that the Prox1 transcription factor is located in the nuclei of the SLYM membrane.
In closing, we would like to take special note of our colleagues’ comments that our study was clinically irresponsible. Quite the contrary, our data serve to inform a diversity of hitherto enigmatic issues, ranging from the variable distributions of subarachnoid hemorrhages spreading, to the inconsistent delivery of intrathecally and intracisternally delivered antisense oligonucleotides, viral vectors, and other biologics to the CNS. Neurosurgeons, who professionally dissect meningeal membranes in live brain have for centuries been aware that the subarachnoid space is divided into multiple compartments. See for example Liliequist, 1956; Yasargil et al., 1976. Our study has already been widely circulated in the neurology and neurosurgery communities, which seem to appreciate the data and their importance.
—Kjeld Møllgård, Christine Delle, all affiliates of the University of Copenhagen, are co-authors of this comment.
References:
Møllgård K, Beinlich FR, Kusk P, Miyakoshi LM, Delle C, Plá V, Hauglund NL, Esmail T, Rasmussen MK, Gomolka RS, Mori Y, Nedergaard M. A mesothelium divides the subarachnoid space into functional compartments. Science. 2023 Jan 6;379(6627):84-88. Epub 2023 Jan 5 PubMed.
Mestre H, Tithof J, Du T, Song W, Peng W, Sweeney AM, Olveda G, Thomas JH, Nedergaard M, Kelley DH. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat Commun. 2018 Nov 19;9(1):4878. PubMed.
Benias PC, Wells RG, Sackey-Aboagye B, Klavan H, Reidy J, Buonocore D, Miranda M, Kornacki S, Wayne M, Carr-Locke DL, Theise ND. Structure and Distribution of an Unrecognized Interstitium in Human Tissues. Sci Rep. 2018 Mar 27;8(1):4947. PubMed.
Nabeshima S, Reese TS, Landis DM, Brightman MW. Junctions in the meninges and marginal glia. J Comp Neurol. 1975 Nov 15;164(2):127-69. PubMed.
Krisch B, Leonhardt H, Oksche A. The meningeal compartments of the median eminence and the cortex. A comparative analysis in the rat. Cell Tissue Res. 1983;228(3):597-640. PubMed.
Krisch B, Leonhardt H, Oksche A. Compartments and perivascular arrangement of the meninges covering the cerebral cortex of the rat. Cell Tissue Res. 1984;238(3):459-74. PubMed.
Brøchner CB, Holst CB, Møllgård K. Outer brain barriers in rat and human development. Front Neurosci. 2015;9:75. Epub 2015 Mar 16 PubMed.
Orlin JR, Osen KK, Hovig T. Subdural compartment in pig: a morphologic study with blood and horseradish peroxidase infused subdurally. Anat Rec. 1991 May;230(1):22-37. PubMed.
Weed LH. Meninges and Cerebrospinal Fluid. J Anat. 1938 Jan;72(Pt 2):181-215. PubMed.
Andres KH. [On the fine structure of the arachnoidea and dura mater of mammals]. Z Zellforsch Mikrosk Anat. 1967;79(2):272-95. PubMed.
Millen JW, Woollam DH. The Anatomy of the Cerebrospinal Fluid. Oxford University Press, 1962 Oxford University Press
LILIEQUIST B. The anatomy of the subarachnoid cisterns. Acta radiol. 1956;46(1-2):61-71. PubMed.
Yasargil MG, Kasdaglis K, Jain KK, Weber HP. Anatomical observations of the subarachnoid cisterns of the brain during surgery. J Neurosurg. 1976 Mar;44(3):298-302. PubMed.
University of Colorado, Anschutz Medical Campus
I read with great interest the recent paper by Møllgård et al. describing a subset of cells in the meninges co-expressing Podoplanin (Pdpn) and Prox1. I have spent over 15 years studying meninges function in brain and vascular development and, more recently, molecular heterogeneity of the meningeal cell layers in the fetal and adult brain. I believe the meningeal layers are functionally specialized to support brain health and function, this based on our work and literature on developing and mature meninges that has been steadily building over the last 20+ years.
In my view, the meninges are too often viewed as a single structure versus its individual parts. Decades of ultrastructural studies, now augmented with single-cell profiling studies in developing and adult meninges, show that there are likely three molecularly distinct dural fibroblast layers (Farmer et al., 2021), at least two arachnoid layers (our work, DeSisto et al., 2020, arachnoid barrier cell, lower arachnoid fibroblasts that express Raldh2/Crabp2 and potentially cells associated with trabeculae that may be molecularly similar) in addition to pia and perivascular fibroblast layers that are molecularly similar but have different locations.
Our validation in human fetal brain (DeSisto et al., 2020), a recent paper (Yang et al., 2022), and a preprint (Kearns et al., 2022) in human adult leptomeninges single-cell profiling support that layer-specific molecular profiles are present in human meninges. We are at the point where it is not accurate to say, “This is a function of the pia. This is a function of the arachnoid. This is a function of the dura.” Adopting additional sublayer-specific descriptors, for example a molecular marker(s) and/or functional aspect like “E-cadherin+ arachnoid barrier cell” or “Lama1+ pial cell” or “Raldh2+ arachnoid cell,” is helpful to advance the meninges biology field. The authors in this paper are bringing the importance of studying meningeal sublayers and their potential functions to the forefront.
That said, I have significant concerns about the disconnect between the anatomical location within the meninges of the Pdpn+/Prox1+ layer (for which the authors provide data from in vivo imaging Prox1-GFP mice and mouse and human histology in sections) and the authors’ claim of functional segregation and barrier within the subarachnoid space by the Pdpn/Prox1 layer. In addition to reading the paper, I have read the authors' rebuttal posted here on Alzforum, which they wrote in response to critiques from knowledgeable scientists who study CNS barriers, CSF production and flow, and the meninges. I support the content of those original critiques by my colleagues.
I provide my own critiques of the Møllgård et al. paper, which are similar to those of my colleagues, but I am taking a different tack. Also, I outline my concerns about the data and the cited literature in the authors' rebuttal that I do not believe lend support to their original claims.
In the interest of making the full text and figures of my comment available, please use this link to the PDF posted on my lab's website.
References:
Farmer DT, Mlcochova H, Zhou Y, Koelling N, Wang G, Ashley N, Bugacov H, Chen HJ, Parvez R, Tseng KC, Merrill AE, Maxson RE Jr, Wilkie AO, Crump JG, Twigg SR. The developing mouse coronal suture at single-cell resolution. Nat Commun. 2021 Aug 10;12(1):4797. PubMed.
DeSisto J, O'Rourke R, Jones HE, Pawlikowski B, Malek AD, Bonney S, Guimiot F, Jones KL, Siegenthaler JA. Single-Cell Transcriptomic Analyses of the Developing Meninges Reveal Meningeal Fibroblast Diversity and Function. Dev Cell. 2020 Jul 6;54(1):43-59.e4. PubMed.
Yang AC, Vest RT, Kern F, Lee DP, Agam M, Maat CA, Losada PM, Chen MB, Schaum N, Khoury N, Toland A, Calcuttawala K, Shin H, Pálovics R, Shin A, Wang EY, Luo J, Gate D, Schulz-Schaeffer WJ, Chu P, Siegenthaler JA, McNerney MW, Keller A, Wyss-Coray T. A human brain vascular atlas reveals diverse mediators of Alzheimer's risk. Nature. 2022 Mar;603(7903):885-892. Epub 2022 Feb 14 PubMed.
Kearns N, Iatrou A, Flood D, De Tissera S, Mullaney ZM, Xu J, Gaiteri C, Bennett DA, Wang Y. Dissecting the Human Leptomeninges at single-cell resolution. bioRxiv. December 18, 2022 bioRxiv
University of Rochester Medical Center
University of Copenhagen
We appreciate Dr. Siegenthaler’s interest in our work. We have previously provided a lengthy explanation outlining the significant fixation artifacts that change not only the morphology, but also the apparent locations, of fragile membranes within fluid-filled spaces—including in this case the membranes within subarachnoid compartments. In addition, we have described how tracers are mislocated upon death, as first reported by Dr. Theise’s group (Benias et al., 2018).
In her critique of our present study, Dr. Siegenthaler refers to histological studies, as well as to the phenotypic characterization of tissues harvested from other species, other developmental stages, and other CNS regions. Yet such imprecise comparisons can be as misleading as they are uninformative. Brain fluid transport and its vectorially-organized flow dynamics are highly specialized, and as such, so are the membranes covering the CNS. It is thus critical to avoid generalization based on fixation artifact, and to instead capitalize upon the availability of contemporary live-imaging technology to accurately describe brain fluid dynamics.
Importantly, these new technologies can validate as well as expand the scope of traditional histology. Indeed, our recent publication demonstrated that imaging of collagen around penetrating arteries using second harmonic generation replicated traditional immunohistochemistry (Mestre et al., 2022), in contrast to Dr. Siegenthaler’s concerns in that regard. Going forward, we hope that Dr. Siegenthaler will participate in the acquisition of new and relevant in vivo data, which might better shed new light on the exciting world of glymphatic transport and its structural restrictions.
References:
Benias PC, Wells RG, Sackey-Aboagye B, Klavan H, Reidy J, Buonocore D, Miranda M, Kornacki S, Wayne M, Carr-Locke DL, Theise ND. Structure and Distribution of an Unrecognized Interstitium in Human Tissues. Sci Rep. 2018 Mar 27;8(1):4947. PubMed.
Mestre H, Verma N, Greene TD, Lin LA, Ladron-de-Guevara A, Sweeney AM, Liu G, Thomas VK, Galloway CA, de Mesy Bentley KL, Nedergaard M, Mehta RI. Periarteriolar spaces modulate cerebrospinal fluid transport into brain and demonstrate altered morphology in aging and Alzheimer's disease. Nat Commun. 2022 Jul 6;13(1):3897. PubMed.
All India Institute of Medical Sciences-Patna
A team of human anatomists, clinicians, and basic scientists have endeavored to examine the controversy over existence of SLYM, see open access document here https://osf.io/5mhtu/?view_only=e5a2bf6004dd498db87587ec8c32df9e
Make a Comment
To make a comment you must login or register.