CSF Proteomic Panel Better Predicts Decline Than Do Classic AD Biomarkers
Quick Links
A panel of 48 CSF proteins—Aβ or tau not among them—distinguished people with Alzheimer's from controls with 94 percent accuracy, according to a study published September 6 in Science Translational Medicine. Adding classic AD biomarkers to this curated panel bumped up accuracy even more. The work was led by Thomas Wingo, Nicholas Seyfried, and Allan Levey at Emory University in Atlanta.
- Panel of 48 CSF proteins performs at least as well as canonical biomarkers in detecting Alzheimer's.
- Combining both predicted decline the best.
- Curiously, CSF Aβ42 and amyloid-PET correlated with different proteomic changes.
The new proteomic panel includes proteins from myriad biological processes involved in AD pathogenesis. It tracked with changes in brain metabolism and volume, and even predicted future cognitive decline. Strikingly, the study illuminated mechanistic differences underlying the standard AD biomarkers. For example, low CSF Aβ42 correlated with changes in synaptic proteins, whereas amyloid-PET scans aligned with proteins in aggregation and metabolism.
“The results are impressive: Not only can the CSF panel of 48 proteins match existing canonical AD biomarkers, but its ability to predict cognitive decline and dementia severity slightly exceeds them,” wrote Gerold Schmitt-Ulms of the University of Toronto.
“This paper continues a series of homeruns by this group,” wrote Russell Swerdlow of Kansas University Medical Center in Kansas City. “Papers like this one illustrate the power of using biomarkers as biologic clues, as opposed simply to answers of what causes AD.”
The data are the latest in a long line of proteomics studies by these Emory scientists. To glean biological insight into disease and discover new biomarkers, they had previously plumbed the depths of brain and CSF proteomes from people across the spectrum of AD (Higginbotham et al., 2020; Feb 2022 news). From these broad analyses, they recently curated a panel of 59 proteins to measure in the CSF of carriers of autosomal-dominant AD mutations (Aug 2023 news on Johnson et al., 2023). Lo and behold, some of these CSF proteins became more or less abundant as early as 30 years before symptoms were expected to start, perhaps even a tad before classic AD biomarkers such as CSF Aβ42 or p-tau217 change. Those findings pointed to different biological processes at play along the path of AD pathogenesis, and nominated new biomarkers and therapeutic targets.
For their current study, first author Rafi Haque and colleagues designed yet another panel, and put it to the test. Would it detect AD? Would it predict cognitive decline? The proteins in this panel were mostly the same as those in the previous study with ADAD mutation carriers, and came from a broad range of synaptic, metabolic, and inflammatory processes that had previously come up for different aspects of AD pathogenesis.
Notably, unlike more cumbersome techniques required for the scientists' larger, unbiased proteomic analyses, this pared-down panel was measured with selective reaction monitoring. This high-throughput technique churns out results in a few minutes, Levey told Alzforum.
The scientists used CSF samples from 706 participants in the AD Neuroimaging Initiative (ADNI), including 220 who were cognitively normal, 376 with a clinical diagnosis of mild cognitive impairment, and 110 with AD dementia. Participants were also grouped into biomarker categories based on measures of CSF Aβ42 and p-tau181. Of the 48 proteins, 18 were significantly elevated, and four reduced, in AD relative to control samples.
The most abundant proteins in AD relative to control CSF were YWHAZ and YWHAB; they by themselves detected AD with 78 percent accuracy. As members of the 14-3-3 family, these proteins have previously been tied to AD and other neurodegenerative diseases. They play a role in many aspects of neuronal function. SMOC1, one of the Aβ-plaque-associated, extracellular matrix proteins collectively known as the matrisome, came in a close second, followed by a host of metabolic proteins. The four proteins that were scarcer in AD samples—VGF, SCG2, NPTXR, and NPTX2—play roles in different aspects of synaptic function.
Collectively, the panel of 48 proteins distinguished AD from control samples with an accuracy of 94 percent, while three standard AD biomarkers—CSF Aβ42, p-tau181, and total tau—together clocked in at 91 percent. Putting both panels together raised the accuracy to 96 percent.
The researchers took stock of numerous associations between the CSF panel and other AD measurements. For example, they found that drops in FDG-PET uptake and hippocampal volume were linked to changes in similar sets of proteins. Oddly, despite the notion that FDG-PET is a measure of brain glucose metabolism, metabolic proteins were not among those tied to this type of scan. Instead, reduction of synaptic proteins and Aβ42, and increased abundance of p-tau181, total tau, and the two 14-3-3 proteins, were most strongly tied to both FDG-PET and hippocampal shrinkage.
An even bigger surprise came when the scientists investigated which proteins on the CSF panel associated with CSF Aβ42 versus amyloid-PET. Considered by most researchers to reflect amyloid deposition in the brain, the two markers are usually 90 percent concordant across studies. However, they were remarkably discordant in their associations with proteins on the 48-protein panel. CSF Aβ42 and florbetapir-PET associated with 21 and 25 protein changes, respectively, but only 11 of those overlapped. Low CSF Aβ42 tied most closely to reduction in synaptic proteins, while florbetapir-PET uptake linked with abundance of SMOC1, 14-3-3, and metabolic proteins.
To the authors, this suggest that although these two measures track closely within a given individual, they may reflect distinct underlying mechanisms. While CSF Aβ42 tracks with changes in synaptic function, amyloid-PET is associated with Aβ aggregation. “Thus, we advocate against using these two biomarkers interchangeably,” the authors wrote.
The situation with tau was the opposite. CSF p-tau181 and total tau are thought to reflect different aspects of AD pathogenesis; even so, both tracked with roughly the same proteomic changes.
To Swerdlow, these findings underscore the importance of exploring the biology of the markers used to define and categorize disease. “For example, data presented here challenge the assumption that plaque absorption of CSF Aβ automatically accounts for reduced AD CSF Aβ levels,” he wrote. “This is consistent with mechanistic studies that show neuronal health alters APP intracellular targeting and Aβ secretion.”
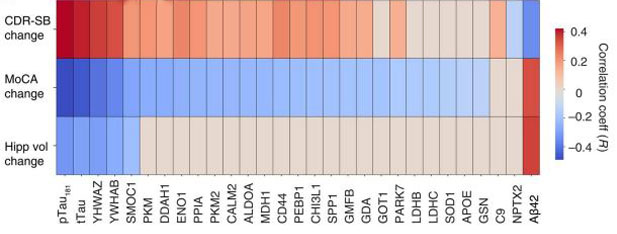
Who Will Get Worse? The abundance of 48 CSF proteins predicted future changes in dementia status, cognitive decline, and hippocampal volume in a research cohort. [Courtesy of Haque et al., Science Translational Medicine, 2023.]
The CSF 48 panel shone the brightest in its association with cognitive impairment and dementia. It outdid a combination of standard AD CSF biomarkers in predicting baseline scores in the Montreal Cognitive Assessment (MoCA) and Clinical Dementia Rating Scale Sum of Boxes (CDR-SB). More importantly to Levey’s mind, it strongly predicted future decline and neurodegeneration. Specifically, the panel outperformed canonical CSF biomarkers in “knowing” who would slip on MoCA and CDR-SB scores, as well as whose hippocampus would shrink among participants who had at least three follow-up assessments (image below). Combining the CSF 48 panel with CSF Aβ42, p-tau181, and total tau further sharpened the predictive value.

Mechanisms Behind Markers. Florbetapir-PET (AV45) and CSF Aβ42 associated with mostly different protein changes. In contrast, CSF p-tau181 and total tau were tied to similar proteomic shifts. [Courtesy of Haque et al., Science Translational Medicine, 2023.]
Predicting cognitive decline is where the panel will likely be most useful, Seyfried told Alzforum. In clinical trials, the CSF 48 panel could help pick out participants who are on the precipice of decline, and/or for tracking biological responses to treatment. Standard CSF biomarkers are great for diagnosis, he said, but not for predicting decline. This is because dozens of other complex processes in the brain contribute to vulnerability versus resilience to disease progression, even between people with the same level of Aβ or tau pathology. “With this panel, we can tap into these other processes that track better with cognitive impairment,” Seyfried said.
James Doecke of CSIRO Brisbane and Colin Masters of the University of Melbourne emphasized the need for better biomarkers. “The challenge has never been greater: advances in therapeutic targeting of Aβ demand better theranostics which will identify responders/nonresponders for aducanumab, lecanemab, and donanemab; and markers of efficacy to provide information on when to stop treatments,” they wrote.
In addition, the 48-protein panel could give memory clinic patients a better idea of what to expect following a diagnosis with standard AD biomarkers, said Jasmeer Chhatwal of Massachusetts General Hospital in Boston. “This is the question my patients most want answered: How fast am I going to decline?” Chhatwal said. He is eager to see it validated in a memory clinic population. Following such validation, this methodology can be scaled up for broader use outside of research cohorts, Levey and Seyfried believe.
Chhatwal previously reported that vascular risk factors are one scourge that colludes with Aβ to hasten cognitive decline. He noted that future studies could assess the extent to which vascular dysfunction, and other modifiable risk factors, contribute to proteomic changes on this panel (May 2018 news).—Jessica Shugart
References
News Citations
- Proteomics Highlight Alzheimer’s Changes in Matrisome, MAPK Signaling
- Proteins in Biofluids Foreshadow Dementia by 30 Years
- Bad Synergy—Together, Vascular Problems and Aβ Hasten Memory Slippage
Paper Citations
- Higginbotham L, Ping L, Dammer EB, Duong DM, Zhou M, Gearing M, Hurst C, Glass JD, Factor SA, Johnson EC, Hajjar I, Lah JJ, Levey AI, Seyfried NT. Integrated proteomics reveals brain-based cerebrospinal fluid biomarkers in asymptomatic and symptomatic Alzheimer's disease. Sci Adv. 2020 Oct;6(43) Print 2020 Oct PubMed.
- Johnson EC, Bian S, Haque RU, Carter EK, Watson CM, Gordon BA, Ping L, Duong DM, Epstein MP, McDade E, Barthélemy NR, Karch CM, Xiong C, Cruchaga C, Perrin RJ, Wingo AP, Wingo TS, Chhatwal JP, Day GS, Noble JM, Berman SB, Martins R, Graff-Radford NR, Schofield PR, Ikeuchi T, Mori H, Levin J, Farlow M, Lah JJ, Haass C, Jucker M, Morris JC, Benzinger TL, Roberts BR, Bateman RJ, Fagan AM, Seyfried NT, Levey AI, Dominantly Inherited Alzheimer Network. Cerebrospinal fluid proteomics define the natural history of autosomal dominant Alzheimer's disease. Nat Med. 2023 Aug;29(8):1979-1988. Epub 2023 Aug 7 PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Haque R, Watson CM, Liu J, Carter EK, Duong DM, Lah JJ, Wingo AP, Roberts BR, Johnson EC, Saykin AJ, Shaw LM, Seyfried NT, Wingo TS, Levey AI. A protein panel in cerebrospinal fluid for diagnostic and predictive assessment of Alzheimer's disease. Sci Transl Med. 2023 Sep 6;15(712):eadg4122. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of Toronto
This paper follows close on the heels of several proteomic studies that interrogated biofluids for predictive AD biomarkers, including groundbreaking work from the Emory group and collaborators themselves.
Whereas in their recent Johnson et al. Nat Neurosci paper, the Emory team and collaborators discovered CSF biomarkers relevant for autosomal-dominant Alzheimer's disease (ADAD), this time the focus was on the diagnostic performance of the group’s selected reaction monitoring (SRM)-based assay (Watson et al., 2023, SciData) for predicting late-onset Alzheimer's disease (LOAD) with samples of individuals aged 55-90 provided by the Alzheimer's Disease Neuroimaging Initiative (ADNI).
The results are impressive: Not only can the CSF panel of 48 proteins match existing canonical AD biomarkers, but its ability to predict cognitive decline and dementia severity slightly exceeds them. There are overlaps to the previous ADAD CSF study but also interesting new insights.
For instance, although the study is not the first to suggest that PET-imaged amyloid deposition in the brain and low diffusible Aβ42 levels in the CSF are not strictly correlated in AD, the authors make a compelling case that the abundances of proteins whose levels are altered in LOAD can correlate with one or the other of these two AD biomarkers, thereby establishing them to be somewhat independent manifestations of AD disease progression. Contrasting this divergence, the same 48 CSF panel proteins correlated equally well with augmented total tau and phospho-tau 181 levels, indicating that these two biomarkers may not be as independent as some prior work has suggested.
The authors dived deeper by tying specific cellular biology to canonical AD biomarkers. For instance, the subset within the 48 CSF panel proteins that correlates best with amyloid deposition measured by PET imaging primarily contributes to the matrisome, 14-3-3 signaling, and cellular metabolism, whereas the sub-pool that correlates with low CSF Aβ42 levels is enriched in synaptic proteins.
It is well-established that the APOE polymorphism plays no significant role in ADAD but has a major effect on disease onset in LOAD. Given this backdrop, I would be curious to learn how the stratification of the ADNI cohorts by APOE genotype would have affected the results. Not surprisingly, relative to the control cohort, the dementia ADNI cohort used in this study was enriched in APOE-ε4 allele carriers, raising the question which, if any, of the proteins in the 48 CSF protein panel may reflect cellular biology governed by the APOE-ε4 polymorphism and to which extent their presence contributed to the diagnostic performance of the SRM assay.
It is unlikely that a complex selective reaction monitoring assay will be adapted widely for routine AD diagnostic. Although such assays may become more broadly implemented in the years to come, in the interim, clinical translation would be eased if the 48 CSF panel was reduced to the subset of proteins whose quantitation alongside canonical biomarkers can best augment the diagnostic performance of widely used immunoassays that quantify Aβ42, total tau, and phospho-tau.
I look forward to seeing this story develop and to hopefully having the Emory group and collaborators present a comparison of their ADAD and LOAD data side-by-side in a future report.
University of Kansas
This paper continues a series of home runs by this group. By organizing measurements of diagnostically informative proteins into larger groups defined through functional or physical relationships, it give us blind men a more global appreciation of the proverbial AD elephant. To me at least, this paper emphasizes how important it is to actually understand the biology of the biomarkers we use to define and categorize the disease.
For example, data presented here challenge the assumption that plaque absorption of CSF Aβ automatically accounts for reduced AD CSF Aβ levels. This is consistent with mechanistic studies that show neuronal health alters APP intracellular targeting and Aβ secretion. It also reveals associations between tau and energy-relevant carbon flux in AD patient CSF, which provides context and perspective to studies that demonstrate this relationship in model systems. Papers like this one by Haque et al. illustrate the power of using biomarkers as biologic clues, as opposed simply to answers, of what causes AD.
References:
Wilkins HM, Troutwine BR, Menta BW, Manley SJ, Strope TA, Lysaker CR, Swerdlow RH. Mitochondrial Membrane Potential Influences Amyloid-β Protein Precursor Localization and Amyloid-β Secretion. J Alzheimers Dis. 2022;85(1):381-394. PubMed.
Weidling IW, Wilkins HM, Koppel SJ, Hutfles L, Wang X, Kalani A, Menta BW, Ryan B, Perez-Ortiz J, Gamblin TC, Swerdlow RH. Mitochondrial DNA Manipulations Affect Tau Oligomerization. J Alzheimers Dis. 2020;77(1):149-163. PubMed.
CSIRO
University of Melbourne
Some of us are old enough to remember the 1986 useful proteomic discovery of a 14-3-3 spot on a two-dimensional gel, which proved to be of diagnostic value for Creutzfeldt-Jakob disease (Harrington et al., 1986). Since then, proteomic approaches on biofluids in the neurodegenerative diseases have had only modest successes, notwithstanding major technical advances in mass spectroscopy. The challenge has never been greater: advances in therapeutic targeting of Aβ-amyloid demand better theranostics which will identify responders/nonresponders for aducanumab, lecanemab, and donanemab; and markers of efficacy to provide information on when to stop treatments.
The paper by Haque and colleagues demonstrates a thorough investigation into a prespecified set of CSF biomarkers compared with classical CSF AD biomarker and their associations with Aβ-amyloid-PET, change in cognition, and change in hippocampal volume. This is certainly an important piece of work, and highlights that identified markers for the soluble fraction of Aβ and Tau are not always the same as markers identified for the insoluble Aβ plaques.
Although we have known for many years that CSF Aβ levels fall as Aβ-PET signals rise, the exact mechanics underlying these phenomena are still obscure. This new work takes this puzzle further, demonstrating that of the CSF 48 panel, 21 markers were associated with CSF Aβ42, whilst 25 markers were associated with Aβ-PET, with only 11 markers intersecting across both. Of interest are the results showing low CSF Aβ42 is associated with a decreased abundance in synaptic proteins, whilst an increase in Aβ-PET was most strongly associated with matrisomal and metabolic proteins.
Certainly, the plethora of statistical analyses here demonstrate associations with preclinical, prodromal, and clinical manifestations of AD. Possibly the low number of associations for hippocampal volume were due to the decreased sample size in this subgroup. Comparative AUC analyses demonstrate that the combination of multiple markers from the CSF 48 panel using penalized logistic regression have strong discriminatory accuracy to separate disease classes, however, adding the canonical markers still improved the AUC values to predict AD, indicating that they contribute additive information on disease status over and above the new CSF 48 panel.
The strength of longitudinal studies such as this, with large sample numbers followed for at least 36 months, provides good evidence of associations between change in cognition and baseline biomarker level. A nice addition to this work will be the in-depth analyses of individual biomarker profiles with both early and late-stage cognitive change using linear mixed effects models, something that was notably missing here.
Overall, this is a great study showcasing advanced proteomic associations with different facets of AD. But the great theranostic challenge remains and looms large.
References:
Harrington MG, Merril CR, Asher DM, Gajdusek DC. Abnormal proteins in the cerebrospinal fluid of patients with Creutzfeldt-Jakob disease. N Engl J Med. 1986 Jul 31;315(5):279-83. PubMed.
Boston University School of Medicine
This selective panel of 48 CSF protein study, found from deep discovery-based proteomics in AD patients defined by Aβ42, tTau, and pTau181 difference compared to control individuals, gives novel insights on biological, cellular process of current biomarkers and significantly improves the estimation of future cognitive decline. The panel is working surprisingly well to predict clinical diagnosis. The prediction significantly improves when this 48 protein panel is added to CSF Aβ and tau biomarkers.
Interestingly, the 48 proteins can best estimate CSF pTau relative to PET amyloid biomarker and are mainly metabolic proteins, suggesting AD phenotypes have a strong implication of brain metabolic dysfunction. Unexpectedly, those metabolic proteins are not a good predictor for cognitive measures. While all proteins are highly correlated with CSF pTau and tTau, there is a distinct pattern of correlation between amyloid PET binding vs. CSF Aβ with 48 proteins. For example, amyloid PET binding has high correlation with the matrisomal protein SMOC1, whereas CSF Aβ correlates with synaptic proteins NPTXR and NPTX2. This result further implicates the utility of protein biomarkers to differentiate patient heterogeneity.
Among others, “SMOC1, a protein previously identified as a hub protein for the matrisomal/extracellular matrix–associated coexpression module” caught my attention. Matrisome pathways are the most significantly upregulated in AD and APOE4 patients in a transcriptome (TCW et al., 2022) and proteome (Johnson et al., 2022) study. Also, SMOC1 is found in the matrisome module of prior proteomic network analysis (Johnson et al., 2022). Of note, only three out of 48 proteins are highly correlated with cognitive measures, and one of three is SMOC1, potentially an important marker for precise diagnosis.
Future studies on identifying more matrisome targets, not only associated with the presence of amyloid and tau pathology but also before the presence of AD pathology, will enable identification of the right treatment window before cognitive decline.
References:
Tcw J, Qian L, Pipalia NH, Chao MJ, Liang SA, Shi Y, Jain BR, Bertelsen SE, Kapoor M, Marcora E, Sikora E, Andrews EJ, Martini AC, Karch CM, Head E, Holtzman DM, Zhang B, Wang M, Maxfield FR, Poon WW, Goate AM. Cholesterol and matrisome pathways dysregulated in astrocytes and microglia. Cell. 2022 Jun 23;185(13):2213-2233.e25. PubMed. BioRxiv.
Johnson EC, Carter EK, Dammer EB, Duong DM, Gerasimov ES, Liu Y, Liu J, Betarbet R, Ping L, Yin L, Serrano GE, Beach TG, Peng J, De Jager PL, Haroutunian V, Zhang B, Gaiteri C, Bennett DA, Gearing M, Wingo TS, Wingo AP, Lah JJ, Levey AI, Seyfried NT. Large-scale deep multi-layer analysis of Alzheimer's disease brain reveals strong proteomic disease-related changes not observed at the RNA level. Nat Neurosci. 2022 Feb;25(2):213-225. Epub 2022 Feb 3 PubMed.
Washington University School of Medicine
This is another fascinating study of CSF proteomics, now in late-onset AD, in parallel to the recent Johnson et al. paper in autosomal-dominant AD. Once again, proteins related to metabolism—particularly glycolysis—showed strong relationships with other AD related biomarkers, including CSF pTau181 and tTau. It will be important to unpack where these metabolic proteins are coming from, including from what cells and why, so as to better understand what they might indicate.
The relatively weak or absent relationship between the metabolic proteins and FDG PET is particularly interesting. However, FDG PET is a complex measure that involves multiple brain regions and networks, oxygen dependent and non-oxygen dependent metabolic pathways, as well as different kinetic parameters, which can be each investigated separately with the appropriate data and analytical methods. How all of this might affect the relationship between CSF proteomics and FDG PET remains an important question that might further our understanding of both these metabolic proteins in the CSF and FDG PET.
The authors should be congratulated on producing this emerging line of research. I am eager to see how similar studies in the future shed further light into the links between brain metabolism and Alzheimer's disease.
University of Lausanne
Impressive work that aligns with previous studies on proteomics CSF analysis for Alzheimer's disease (AD). The use of panels, which combine signatures from various proteins, could potentially be the key to improving the accuracy of AD diagnosis, monitoring, and early tracking of cognitive decline. What could make it even more intriguing is investigating these correlations in other biofluids such as plasma, which is essential in resource-limited countries for accessible AD diagnoses worldwide and extending clinical trial to diverse populations.
Make a Comment
To make a comment you must login or register.