In Amyloid and Tangle Models, APOE4 Paralyzes Microglia
Quick Links
Though APOE4 is the strongest genetic risk factor for sporadic Alzheimer’s disease, scientists have struggled for decades to figure out how it affects the cells of the brain. For microglia, at least, there may be some clarity. In the September 25 Nature Immunology, researchers led by Oleg Butovsky of Brigham and Women’s Hospital in Boston reported that, in mice, APOE4 causes microglia to ramp up the expression of homeostatic genes. This prevents the cells from clearing amyloid plaques and neurofibrillary tangles. Deleting APOE4, or PU.1, a master driver of microglial homeostasis, enabled the microglia to clear plaques and they recruited astrocytes to help.
- APOE4 locks microglia in a state of homeostasis.
- The microglia do not clear plaque or tangles.
- Deleting APOE4 puts microglia back on track.
In the same journal on October 19, Chia-Chen Liu, Guojun Bu, and colleagues at Mayo Clinic, Jacksonville, Florida, also reported that APOE4 prevents microglia from mopping up plaques in mice. Plus, they found that in brain tissue from people who had had AD, fewer activated microglia surrounded plaques if the donor was an APOE4 carrier.
“Both papers demonstrate conclusively that microglial APOE contributes to AD pathology,” wrote Priyanka Narayan at the National Institutes of Health in Bethesda, Maryland (comment below).
Microglia that flock to and digest amyloid plaques and other neurodegenerative debris have been dubbed a variety of names, including disease-associated microglia (DAMs) or neurodegenerative microglia (MGnDs; Jun 2017 news; Sep 2017 news). These cells upregulate a host of genes, including APOE, but, because astrocytes make most of the APOE in the brain, the contribution of the microglial protein to pathology was unclear.
To get a better idea, the two groups took different approaches. Working with Bu, who has since moved to the Hong Kong University of Science and Technology, co-first authors Liu and Na Wang crossed APP/PS1 mice with mice that have no endogenous APOE and that conditionally overexpress human APOE3 or E4 only in their microglia. In contrast, co-first authors Zhuoran Yin and Neta Rosenzweig in Butovsky’s group crossed human APOE3 or APOE4 knock-in mice with P301S or APP/PS1 animals, then conditionally knocked APOE out of microglia in the offspring. Both groups assessed single-cell transcriptomics of mouse brain tissue, taken when the animals had widespread plaques or tangles.
What happened when microglia expressed APOE? Compared to APP/PS1 or P301S APOE3 mice, those expressing APOE4, be it full-body or just in their microglia, had more homeostatic cells and fewer DAMs/MGnDs. ApoE4 microglia upregulated homeostatic drivers, including TGFβ and PU.1. Genetic variants near PU.1 reportedly increase its expression and delay AD onset, potentially by suppressing homeostatic genes (Aug 2019 news; Jun 2017 news). After treating microglia from APOE4 knock-ins with a PU.1 inhibitor, the cells began expressing DAM/MGnDs genes.
Yin and Rosenzweig found that knocking out microglial APOE4 seemed to reinvigorate the cells. The mice mobilized as many DAMs/MGnDs as did their APOE3 counterparts (image below). These results suggest that APOE4 microglia only muster a weak response to AD pathology.
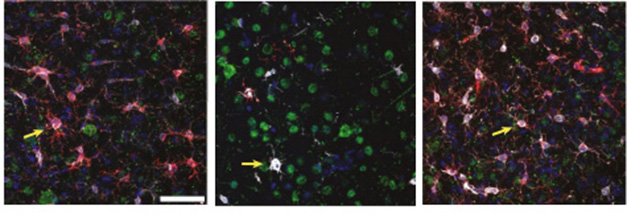
APOE Flips the Switch. In E3/P301S knock-in mice (left), microglia expressing the MGnD marker CLEC7A (red) swarm aggregates of phosphorylated tau (green). Microglia from E4 knock-ins (middle) only muster a weak response to pathology. Knocking out APOE4 in microglia restores their plaque response (right). Arrows show CLEC7A-positive microglia associated with p-tau. [Courtesy of Yin et al., Nature Immunology, 2023.]
What effect does microglial APOE4 have on pathology? APOE4/P301S mice had twice as many phospho-tau aggregates and 25 percent more cortical neuron degeneration than their E3/P301S counterparts. Deleting microglial APOE4 reduced both to levels seen in E3/P301S mice.
What about amyloid plaques? In APP/PS1 mice, Butovsky and Bu saw complementary changes in the APOE knock-in and overexpression mice. APOE4 knock-ins, and animals with just E4-expressing microglia, sprouted more amyloid plaques and more dystrophic neurites, but had fewer DAMs/MGnDs than E3/APP/PS1 mice. Bu noted that APOE4 microglia had less intracellular Aβ than did E3 microglia, suggesting impaired plaque phagocytosis. Butovsky saw that deleting APOE4 in microglia stirred them to transition into DAMs/MGnDs, flock to plaques, and reduce the plaque load to that seen in E3/APPPS1 mice.
Using in vivo two-photon imaging, Bu and colleagues also saw that APOE3 microglia, but not E4, surrounded and devoured plaques, extending cellular processes to intertwine with plaque contents. E4 microglia, on the other hand, barely moved (image below). Their poor response correlated with worse synaptic function and poorer memory in the E4 mice.

Feeling Their Way. Within a mouse brain, APOE3 microglia (green, left) reach processes towards amyloid plaques (blue), while APOE4 microglia (right) barely move. [Courtesy of Liu et al., Nature Immunology, 2023.]
APOE isoforms controlled more than just microglial activation—they also dictated microglia and astrocyte conversations. Yin and Rosenzweig analyzed cell-surface ligand-receptor pairs expressed by the cells. APOE4 microglia, but not E3 glia, repressed expression of LGALS3, the gene for galectin-3, a protein that regulates immune function and regulation. When they deleted APOE4, LGALS3 became the top ligand interacting with astrocyte receptors. This hints that galectin-3 might be important in astrocyte recruitment to plaques and tangles. Indeed, when the scientists injected recombinant galectin-3 into the brains of APP/PS1 mice, astrocytes flocked to amyloid plaques and plaque load near the injection site fell by one-third over two weeks. To Butovsky, these results suggest that microglia beckon astrocytes to devour plaques and that APOE4 prevents this crosstalk.
Along these lines, Butovsky measured fewer activated astrocytes in APOE4 knock-ins. However, Bu found more of them when microglia overexpressed ApoE4. Emile Wogram and Marco Prinz, both at the University of Freiburg, Germany, chalked up this discrepancy to differences in methods. Bu measured glial fibrillary acidic protein (GFAP) expression throughout the whole mouse cortex, while Butovsky just looked around amyloid plaques. “If both studies had measured GFAP expression specifically around Aβ plaques, their comparisons might have led to similar conclusions,” Wogram and Prinz wrote in an accompanying News and Views article.
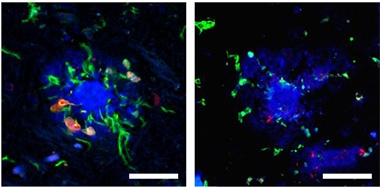
Active E3. Sluggish E4. In brain tissue from an APOE3 carrier (left), microglia (green) expressing the DAM/MGnD marker Lgals3 (red) surround an amyloid plaque (blue). In tissue from an APOE4 AD case (right), microglia are not activated and barely notice the plaque. [Courtesy of Liu et al., Nature Immunology, 2023.]
Would these isoform effects hold in humans? Butovsky and Bu analyzed cortical tissue from people who had AD and who carried either two copies of APOE3 or were heterozygous for APOE4. Both scientists came to the same conclusions. Microglia from APOE4 carriers expressed fewer DAM/MGnD genes, including LGALS3, than cells from E3 carriers. Likewise, fewer Lgals3-positive DAMs/MGnDs surrounded amyloid plaques in tissue slices from APOE4 carriers (image above). Butovsky also detected greater expression of TGFβ signaling and other homeostatic genes in microglia from the E4 carriers, suggesting they are stuck in homeostasis.
All told, both groups of researchers propose a gain of toxic function for APOE4 in microglia, where it acts as a negative feedback regulator to keep the cells homeostatic and quash their response to AD pathology.—Chelsea Weidman Burke
References
Mutations Citations
News Citations
- Hot DAM: Specific Microglia Engulf Plaques
- ApoE and Trem2 Flip a Microglial Switch in Neurodegenerative Disease
- AD Genetic Risk Tied to Changes in Microglial Gene Expression
- Microglial Master Regulator Tunes AD Risk Gene Expression, Age of Onset
Research Models Citations
Other Citations
Further Reading
No Available Further Reading
Primary Papers
- Yin Z, Rosenzweig N, Kleemann KL, Zhang X, Brandão W, Margeta MA, Schroeder C, Sivanathan KN, Silveira S, Gauthier C, Mallah D, Pitts KM, Durao A, Herron S, Shorey H, Cheng Y, Barry JL, Krishnan RK, Wakelin S, Rhee J, Yung A, Aronchik M, Wang C, Jain N, Bao X, Gerrits E, Brouwer N, Deik A, Tenen DG, Ikezu T, Santander NG, McKinsey GL, Baufeld C, Sheppard D, Krasemann S, Nowarski R, Eggen BJ, Clish C, Tanzi RE, Madore C, Arnold TD, Holtzman DM, Butovsky O. APOE4 impairs the microglial response in Alzheimer's disease by inducing TGFβ-mediated checkpoints. Nat Immunol. 2023 Nov;24(11):1839-1853. Epub 2023 Sep 25 PubMed.
- Liu CC, Wang N, Chen Y, Inoue Y, Shue F, Ren Y, Wang M, Qiao W, Ikezu TC, Li Z, Zhao J, Martens Y, Doss SV, Rosenberg CL, Jeevaratnam S, Jia L, Raulin AC, Qi F, Zhu Y, Alnobani A, Knight J, Chen Y, Linares C, Kurti A, Fryer JD, Zhang B, Wu LJ, Kim BY, Bu G. Cell-autonomous effects of APOE4 in restricting microglial response in brain homeostasis and Alzheimer's disease. Nat Immunol. 2023 Nov;24(11):1854-1866. Epub 2023 Oct 19 PubMed.
- Wogram E, Prinz M. APOE set the microglia free. Nat Immunol. 2023 Nov;24(11):1790-1791. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
National Institutes of Health
Variants of APOE have been known for years to influence disease risk and progression. However, teasing apart the cell types that are important to APOE’s effects has remained a challenge. Papers using iPSC-derived cell types in monoculture, co-culture, and complex multicellular cultures have tried to tackle this challenge and have made some key discoveries about cellular effects of APOE alleles; APOE4 disrupts intracellular lipid homeostasis in human iPSC-derived glia (Lin et al., 2018; Sienski et al., 2021; Narayan et al., 2020; Victor et al., 2022; Koutsodendris et al., 2023; Blanchard et al., 2020; Blanchard et al., 2022) however, the broader tissue, pathological, and organismal effects remain elusive. Moreover, it is unlikely that cells in an incubator will capture all the nuances of the mouse brain environment. This is especially important for cells that sense, and adapt to, their environment constantly, such as microglia. These twin papers both explore the role of microglial APOE isoforms in the context of neurodegenerative disease models of amyloid disease and tauopathy.
The two papers take different approaches, each providing their own insights. Liu and Wang et al. use a microglial-specific knock-in of the human APOE3 or APOE4 alleles in the context of an APOE-/- background, whereas Yin and Rosenzweig et al. specifically deleted only microglial APOE in the context of the humanized knock-in APOE mouse model.
Both papers demonstrate conclusively that microglial APOE does contribute to pathology. Both papers acknowledge the involvement of other cell types as well, with special focus on the interplay between astrocytes and microglia (in Yin and Rosenzweig et al.). Both papers perform extensive transcriptomic characterization to show that APOE isoforms alter the transcriptional state(s) of microglia.
From my perspective, it was a nice reinforcement of our initial observation in iPSC culture (Sienski et al., 2021), as well as that of others (Victor et al., 2022; Blanchard et al., 2022; Haney et al., 2023).
to see that in so many different mouse systems, APOE4 correlates with lipid droplet accumulation in microglia! Additionally, as a member of the iNDI team, it is heartening to see Liu and Wang et al. utilize microglia derived from the iNDI isogenic APOE lines to support findings from their mouse studies (see May 2021 news on iNDI).
The differences in mouse models led to some intriguing observations. Liu and Wang et al. noted slightly different effects of APOE3 and APOE4 when expressed in an APOE-/- versus a background with murine ApoE. This draws attention to the fact that murine ApoE changes the role of human APOE expression in a variant-specific manner. Yin and Rosenzweig et al. noted sex differences in their outcomes, suggesting that microglial responses, especially APOE-dependent ones, may be influenced by sex of the organism.
Microglial APOE has a role to play in disease pathology, and APOE4 is worse for pathology than APOE3. Both studies show us microglia are important, but other cell types are, too. The interplay of these cell types, the APOE genotype, and environmental factors may initiate or exacerbate progression of AD.
References:
Lin YT, Seo J, Gao F, Feldman HM, Wen HL, Penney J, Cam HP, Gjoneska E, Raja WK, Cheng J, Rueda R, Kritskiy O, Abdurrob F, Peng Z, Milo B, Yu CJ, Elmsaouri S, Dey D, Ko T, Yankner BA, Tsai LH. APOE4 Causes Widespread Molecular and Cellular Alterations Associated with Alzheimer's Disease Phenotypes in Human iPSC-Derived Brain Cell Types. Neuron. 2018 Jun 27;98(6):1141-1154.e7. Epub 2018 May 31 PubMed.
Sienski G, Narayan P, Bonner JM, Kory N, Boland S, Arczewska AA, Ralvenius WT, Akay L, Lockshin E, He L, Milo B, Graziosi A, Baru V, Lewis CA, Kellis M, Sabatini DM, Tsai LH, Lindquist S. APOE4 disrupts intracellular lipid homeostasis in human iPSC-derived glia. Sci Transl Med. 2021 Mar 3;13(583) PubMed.
Narayan P, Sienski G, Bonner JM, Lin YT, Seo J, Baru V, Haque A, Milo B, Akay LA, Graziosi A, Freyzon Y, Landgraf D, Hesse WR, Valastyan J, Barrasa MI, Tsai LH, Lindquist S. PICALM Rescues Endocytic Defects Caused by the Alzheimer's Disease Risk Factor APOE4. Cell Rep. 2020 Oct 6;33(1):108224. PubMed.
Victor MB, Leary N, Luna X, Meharena HS, Scannail AN, Bozzelli PL, Samaan G, Murdock MH, von Maydell D, Effenberger AH, Cerit O, Wen HL, Liu L, Welch G, Bonner M, Tsai LH. Lipid accumulation induced by APOE4 impairs microglial surveillance of neuronal-network activity. Cell Stem Cell. 2022 Aug 4;29(8):1197-1212.e8. PubMed.
Koutsodendris N, Blumenfeld J, Agrawal A, Traglia M, Grone B, Zilberter M, Yip O, Rao A, Nelson MR, Hao Y, Thomas R, Yoon SY, Arriola P, Huang Y. Neuronal APOE4 removal protects against tau-mediated gliosis, neurodegeneration and myelin deficits. Nat Aging. 2023 Mar;3(3):275-296. Epub 2023 Feb 20 PubMed.
Blanchard JW, Bula M, Davila-Velderrain J, Akay LA, Zhu L, Frank A, Victor MB, Bonner JM, Mathys H, Lin YT, Ko T, Bennett DA, Cam HP, Kellis M, Tsai LH. Reconstruction of the human blood-brain barrier in vitro reveals a pathogenic mechanism of APOE4 in pericytes. Nat Med. 2020 Jun;26(6):952-963. Epub 2020 Jun 8 PubMed. Correction.
Blanchard JW, Akay LA, Davila-Velderrain J, von Maydell D, Mathys H, Davidson SM, Effenberger A, Chen CY, Maner-Smith K, Hajjar I, Ortlund EA, Bula M, Agbas E, Ng A, Jiang X, Kahn M, Blanco-Duque C, Lavoie N, Liu L, Reyes R, Lin YT, Ko T, R'Bibo L, Ralvenius WT, Bennett DA, Cam HP, Kellis M, Tsai LH. APOE4 impairs myelination via cholesterol dysregulation in oligodendrocytes. Nature. 2022 Nov;611(7937):769-779. Epub 2022 Nov 16 PubMed.
Farmer BC, Kluemper J, Johnson LA. Apolipoprotein E4 Alters Astrocyte Fatty Acid Metabolism and Lipid Droplet Formation. Cells. 2019 Feb 20;8(2) PubMed.
Haney MS, Pálovics R, Munson CN, Long C, Johansson P, Yip O, Dong W, Rawat E, West E, Schlachetzki JC, Tsai A, Guldner IH, Lamichhane BS, Smith A, Schaum N, Calcuttawala K, Shin A, Wang YH, Wang C, Koutsodendris N, Serrano GE, Beach TG, Reiman EM, Glass CK, Abu-Remaileh M, Enejder A, Huang Y, Wyss-Coray T. APOE4/4 is linked to damaging lipid droplets in Alzheimer's microglia. bioRxiv. 2023 Jul 25; PubMed.
Make a Comment
To make a comment you must login or register.