Treat Before ‘Aβ Bothers Tau,’ Scientists Say at CTAD
Quick Links
Some researchers have long argued for starting amyloid immunotherapy early, before tangles spread and neurons die all over the brain. At the 16th Clinical Trials on Alzheimer’s Disease conference, held October 24 to 27 in Boston and online, they added flesh to the bone of this idea. Despite coming from different antibodies—donanemab, lecanemab, gantenerumab—the findings paint a convergent picture. In short, participants at the earliest stages of the respective cohorts enrolled in each trial gained the most cognitively from treatment. The findings are preliminary, often involving post hoc analyses of small numbers of participants remaining from large trials. Even so, they stirred excitement in Boston.
- On donanemab, younger people with fewer tangles benefitted the most.
- On lecanemab, too, people with the fewest tangles improved most on CDR-SB.
- In the DIAN prevention trial, eight years of gantenerumab halved disease progression.
In one striking tease, about two-thirds of participants with very early AD who took lecanemab actually improved on the CDR-SB over 18 months, compared with about one-third of a matched placebo group. Other findings offered the first concrete indication that amyloid immunotherapy may be able to prevent AD dementia. In the Dominantly Inherited Alzheimer Network secondary prevention trial, presymptomatic mutation carriers taking gantenerumab for eight years had half the odds of developing symptoms as did those on placebo.
“I think we will deliver on the promise of changing the course of the disease. That day is coming,” Randall Bateman of Washington University in St. Louis, who leads the DIAN Trials Unit, said after accepting this year’s CTAD Lifetime Achievement Award. As Reisa Sperling of Boston’s Brigham and Women’s Hospital quipped, “Who knew? Maybe earlier is better.”
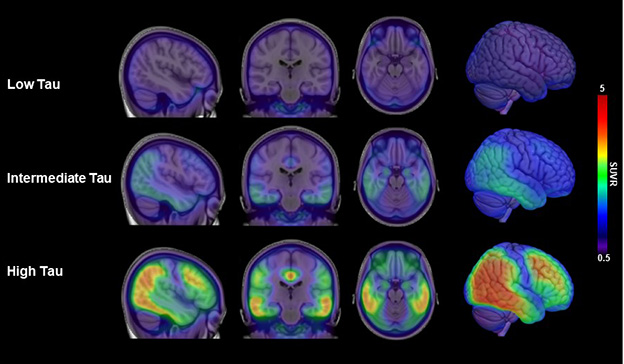
Tangle Load Is Key. People with few tangles, below 1.10 tau PET SUVR (top row) fared better on amyloid immunotherapy than did those with intermediate (middle, 1.10 to 1.46 SUVR) and high (bottom) tangle loads. [Courtesy of Eisai.]
On Donanemab, Few Tangles, Young Age Independently Boost Efficacy
Pharma-led Phase 3 trials for all the large antibody programs have enrolled people with mild cognitive impairment or mild dementia due to AD, leaving open the question of whether the drugs might work better at even earlier stages. To guesstimate, researchers are parsing the data they have. In other words, they are comparing efficacy in people at the earliest-stage and the latest-stage ends of the cohorts they enrolled. Modeling is involved, too.
Mark Mintun of Eli Lilly presented one such post hoc analysis from Trailblazer-Alz2. This Phase 3 study of donanemab used tau PET screening to select people with intermediate tau levels, defined as 1.10 to 1.46 SUVR, for the primary analysis population. The trial also enrolled people with higher loads, to gather data on how that group responded. Because of this, all 1,736 participants, not just a substudy, had baseline tau PET scans. Lilly researchers reasoned that the intensity of the tau PET signal could serve as a proxy for disease stage, an assumption that has generated praise but also questions about why the company did not formally test donanemab against placebo in amyloid-positive people below 1.1 tau PET SUVR.

Fewer Tangles, Better Results. Modeling based on donanemab Phase 3 data predicts that people with the lowest tau PET SUVRs (x axis) will maintain their cognitive abilities the best (black bars). [Courtesy of Eli Lilly.]
When Lilly scientists correlated baseline tangle load with slowing of cognitive decline for each person, they found an inverse relationship. The fewer tangles a person had at the outset, the better that person maintained his or her cognitive abilities while on donanemab. Modeling based on these data predicted that for people with a tau SUVR of 1, cognitive decline would slow by 60 percent on the iADRS and 45 percent on the CDR-SB. At an SUVR of 2, by contrast, those numbers would be as low as 10 and 20 percent (see image above). This compares with measured values of 35 and 36 percent in the intermediate-tau trial population.
Results were similar when researchers measured disease stage in another way, by baseline plasma p-tau217. In the lowest tertile, donanemab worked better than in higher tertiles.
What about even earlier disease stages? In Boston, Mintun said that 1,053 people who did not meet trial screening criteria, due to having tau PET scans below 1.10, were enrolled into an open-label “addendum” study. There was no placebo control for these participants, hence the researchers could not calculate slowing of cognitive decline on donanemab. Even so, biomarker outcomes echoed those for other treated groups, with a rapid drop in amyloid plaques, plasma p-tau217, and the inflammatory marker GFAP. The data hint that this very-low-tau group would also benefit cognitively from treatment, Mintun said, though that remains to be shown.
Besides tangles, the researchers found that a participant’s age independently affected how well they fared on donanemab. Among the half of participants with lower p-tau217 at baseline, those younger than the trial’s median age of 75 slipped 43 percent less on the iADRS than did matched placebo controls. Those 75 or older benefitted less, slipping 30 percent less. For people in the upper half of baseline p-tau217, age made even more difference. Those younger than 75 had a 30 percent slowing of decline, those older, only 8 percent. Age and tangle load have additive effects on efficacy, Mintun concluded.
Because people joined this trial at different points in their disease, the whole cohort encompassed about 10 years of AD pathogenesis, Mintun noted. Lilly researchers used their data to model how a person’s disease stage at baseline might affect their drug response. The model predicted dramatic stage-based differences. For example: People who were two years less advanced than the cohort average could achieve as much as 88 percent slowing of decline on the iADRS, almost stalling their disease. On the other hand, those two years more advanced than the cohort average would gain but a negligible benefit of 6 percent slowing. One reason for this stark difference is that cognitive decline accelerates as disease advances, Mintun said.
“This gives us great urgency in thinking about how to diagnose and prepare patients for treatment in the future,” Mintun concluded.
Lecanemab: Did I Hear “Improvement”?
These donanemab analyses jibe with data from the Phase 3 Clarity trial of lecanemab. In Boston, Keith Johnson of Massachusetts General Hospital, Boston, presented an exploratory analysis relating the baseline tau PET signal in Clarity to its outcome. Johnson divided the trial’s tau PET substudy of 342 people into three groups: low tau, defined as below 1.06 SUVR; medium tau, 1.06 to 2.91; and high tau, above 2.91. These values were chosen based on the different patterns of tangle accumulation they represent: entorhinal cortex and hippocampus for the low-tangle group, those plus limbic regions for medium-tangle, those plus neocortex for high (see image at top of story). There were 141 people in low, 191 in intermediate, and 10 in the high groups, respectively. The latter two groups were combined for analysis. In each group, about half were on drug, half on placebo.
Because Clarity participants were not screened by tau PET, the cohort included a broader range of tangle accumulation than did Trailblazer-Alz2. The low-tau group in particular has not been analyzed in previous antibody trials, Johnson noted. Stephen Salloway of Butler Hospital, Providence, Rhode Island, asked whether this group had different clinical characteristics from higher tau cohorts at baseline. Johnson said, to his surprise, they did not.
How did their outcomes differ? On amyloid plaque clearance, the low-tangle group had much lower baseline amyloid than the intermediate/high group, 36 versus 94 centiloids. Because of this, they cleared plaque faster, falling below the threshold for amyloid positivity, here set at 30 centiloids, three months into the trial. This compared with 18 months for the intermediate/high group. At the individual level, 93 percent of low-tangle participants were amyloid-PET negative by the end of the study, compared with 57 percent of the higher-tangle group.
On tau biomarkers, however, treatment effects were bigger in the intermediate- than low-tangle participants. In the high-tangle group, plasma p-tau181 dropped by twice as much as in low-tau participants, and tangle progression slowed across every brain region examined. In low-tau participants, progression slowed only in medial temporal regions, likely because tangles had not reached the other regions yet.
What about cognition? On placebo, the low-tangle group lost less ground than higher-tangle groups, staying stable on the CDR-SB and slipping only slightly on the ADAS-Cog14 and ADCS MCI-ADLs over 18 months. In contrast, low-tangle participants on lecanemab stayed basically stable on the latter two measures, and bettered their baseline scores on the CDR-SB. Improvement was defined as scoring at least 0.5 points higher than before.
Overall, 76 percent of low-tau participants on lecanemab, and 55 percent on placebo, held their ground on the CDR-SB over 18 months. Remarkably, 60 percent of the cohort improved over baseline, compared with 28 percent of those on placebo. Percentages for the other two cognitive tests were similar. Johnson did not show cognitive data for the intermediate/high tau group alone.
The percentage who improved on drug grew over the course of the trial, agreeing with other data suggesting cognitive benefits take a few months to appear. The number of participants in these groups was small, with only about 50 people on lecanemab having 18-month data. Because of this, p values for many differences were nominal.
The findings caused a stir in the room and later in the hallways. One audience member asked what might account for improving cognitive scores. Michael Irizarry at Eisai speculated that it could be due to the mechanism of action, for example, lecanemab clearing protofibrils that were harming synaptic function. Sperling suggested that removing protofibrils before they have “bothered tau” in the early entorhinal/hippocampal areas might forestall tangle spread into further areas of the brain.
For her part, Sperling presented 24-month open-label extension data. In the OLE, low-tangle participants who switched from placebo to lecanemab appeared to stabilize on cognitive tests, losing no ground between 18 and 24 months. By contrast, the treatment group who had been on lecanemab since the beginning of the trial slipped on all three tests at 24 months relative to 18 months, nearly to the scores of the former placebo group. This type of convergence between groups would be expected for a symptomatic therapy, but not a disease-modifying one. Sperling noted that an early glimpse at 30-month data suggests the two groups are separating again; however, only about half the cohort has reached this time point.

Improving Cognition? On lecanemab (green), more people in the low-tau group stayed stable (left) or improved (right) on the CDR-SB over time than did those on placebo (black). Those who switched from lecanemab to placebo at 18 months also benefitted. [Courtesy of Eisai.]
By the numbers, 79 percent of the low-tau treatment group had not declined on the CDR-SB after 24 months; 50 percent had improved. For the group formerly on placebo, the percentage with no decline on the CDR-SB rose from 55 percent at 18 months to 62 percent at 24 once on lecanemab, and the percentage who improved rose from 28 to 40 percent (see image above).
Only a subset of the Clarity cohort had tau PET scans. To examine a larger group, Sperling used baseline amyloid of below 60 centiloids as a proxy for low tau. She chose this threshold because it encompassed 80 percent of the low-tangle participants. In this larger analysis of below-60 centiloid Clarity participants, the 190 who got lecanemab fared better on cognitive outcomes than did the 190 on placebo, declining by 51, 69, and 72 percent less on the CDR-SB, ADAS-Cog14, and ADCS MCI-ADL, respectively. For the whole Clarity cohort, i.e., low and medium/high tangles combined, these numbers were 27, 26, and 37 percent, again demonstrating more gain earlier in disease. Sperling did not show cognitive data for participants whose baseline amyloid was above 60 centiloids. Logic would demand that their cognitive benefit was below that of the combined group.
Altogether, Sperling said, the data support testing lecanemab in preclinical populations. This is being done in the AHEAD 3-45 and Trailblazer-Alz3 studies. Sperling noted that people with few tangles probably have less neuronal damage than those later in disease. “It’s encouraging that if you get there before neurons are dying, maybe you really can improve function,” Sperling said.
Tiny Shoots of Prevention Sprouting from the Data
Though these Phase 3 donanemab and lecanemab data show promise for earlier stages, all participants already had symptoms of Alzheimer’s dementia. What about catching people while they are still asymptomatic? Bateman offered a glimpse at such a future.
The DIAN Trials Unit secondary prevention study lasted 11 years, from 2012 to 2023. The Phase 2/3 study tested gantenerumab or solanezumab against a pooled placebo group. The double-blind period ended in 2019 with a negative result. Asymptomatic participants had not declined cognitively, thus no treatment effect could be measured. However, gantenerumab had strongly affected biomarkers including cerebrospinal fluid p-tau181 and total tau (Feb 2020 news; Apr 2020 news).
For this reason, the researchers offered gantenerumab to all participants in an open-label extension that lasted until August 2023 (see DIAN research update). For most participants, this meant a treatment gap of about two years was followed by two years of OLE. Of the 73 mutation carriers who completed the OLE, 22 were on gantenerumab for about eight years of exposure, while the remainder had switched from solanezumab or placebo. Bateman noted that the gantenerumab dose had been boosted fivefold during the trial, and was increased another threefold in the OLE. This is because initial trial results showed plaque is harder to budge in mutation carriers, who produce more Aβ, than in late-onset AD patients.
A pre-specified subgroup analysis of the people who were on gantenerumab throughout found they were half as likely to progress to symptoms as were those who switched from placebo or solanezumab. Those who did develop symptoms did so six years later than their expected age of onset (EYO). Curiously, people initially on placebo or solanezumab also had a delay in symptom onset, three years beyond their EYO. In the DIAN observational study, by contrast, symptoms started at the expected age. Because many participants remain asymptomatic, the number of people in this analysis was tiny: nine on gantenerumab, four on solanezumab, and seven on placebo. The findings are preliminary; the researchers are currently doing sensitivity analyses.
Pierre Tariot of the Banner Alzheimer Institute in Phoenix asked if these findings would generalize to late-onset AD. Bateman believes they will, because immunotherapy results for several antibodies have been similar in autosomal-dominant and late-onset populations. He said DIAN participants will continue to receive treatment with amyloid-lowering drugs, but did not say which drugs will be selected next.—Madolyn Bowman Rogers
References
Therapeutics Citations
News Citations
- Topline Result for First DIAN-TU Clinical Trial: Negative on Primary
- In DIAN-TU, Gantenerumab Brings Down Tau. By a Lot. Open Extension Planned
External Citations
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.