Data Fabrication Ousted NIA Neuroscience Director Eliezer Masliah
Quick Links
A top researcher’s fall from grace sends shock waves through the scientific community. Such is the case with neuropathologist Eliezer Masliah, who stands accused of image falsification spanning 26 years. Masliah has published more than 800 papers. He is one of the world’s leading experts on α-synuclein, a protein involved in Alzheimer’s and Parkinson's diseases and the target of multiple drugs in clinical trials based in part on his research.
- Masliah’s preclinical data have supported clinical trials targeting α-synuclein.
- Many micrographs show up multiple times under different labels, some in multiple publications; many western blots appear spliced.
- Masliah may have acted alone, but scores of co-authors now question their collaborations.
- Institutions that employed Masliah and his main collaborators have refused to answer questions about the matter.
On September 26, 2024, the National Institute on Aging (NIA) announced that Masliah had been removed from his position as scientific director of the institute’s neuroscience division after a nine-month-long NIH investigation found “falsification and/or fabrication involving re-use and relabel of figure panels representing different experimental results in two publications.”
The same day, Science magazine posted an investigative story in which reporter Charles Piller and a team of image analysts compiled a 286-page dossier of 132 papers that appear to contain duplicated or doctored images and figures.
Alzforum has reviewed the dossier. It makes for wrenching reading. It details how figures in each paper—sometimes one, sometimes three or more—may have been manipulated, often to show the same information under different labels. The dossier’s authors have posted all the figures as comments on the PubPeer website. Masliah is the only co-author who appears on all the questioned papers. They run from 1997 to 2023, years Masliah was employed at the University of California, San Diego, and NIA in Bethesda, Maryland.
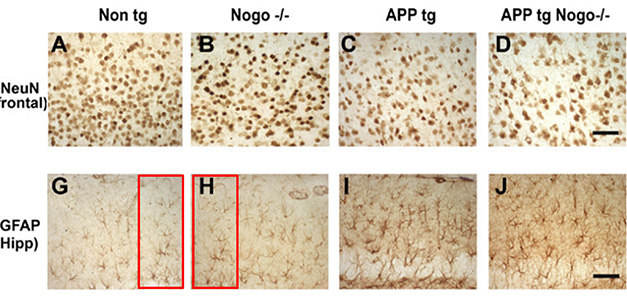
Reuse, Recycle? Mouse hippocampi seem to contain the same image cropped differently and published as being from different samples. Panel G is labeled as coming from a non-transgenic mouse, whereas H is purportedly from a Nogo homozygous knockout. Several dozen papers were flagged for this type of problem. PubPeer.
On September 23, 2024, three days before his removal and the Science article, Masliah opened the 2024 NIH Alzheimer’s Research Summit, speaking after NIA director Richard Hodes. The NIH did not respond to questions about whether the conference organizers knew Masliah was under an investigation that had concluded on September 15, 2024.
Two weeks after the allegations first appeared, the neurodegeneration research community is still reeling with disbelief. Many scientists spoke with Alzforum but were unwilling to be quoted. In essence, they said that the extent of the manipulation took their breath away and made them deeply concerned for the reputation of scientific investigation in their field.
Already, the situation has drawn the eye of a U.S. congressional committee that is urging greater transparency and other reforms at the NIH. “These findings are deeply troubling and worsen an already growing distrust of the NIH’s scientific research,” Cathy McMorris Rodgers (R-WA), chair of the House Energy and Commerce Committee, along with two subcommittee chairs, said in a statement.
Masliah has not commented publicly and did not respond to an interview request from Alzforum. The NIH, responding on behalf of NIA, said it “does not discuss personnel matters” when asked whether Masliah is still employed at the institute. A spokesperson at UCSD, where Masliah worked between 1990 and 2016 before moving to NIA, would not confirm whether it is conducting its own investigation but said it “addresses all allegations in accordance with university policy and procedures.”
Meanwhile, concern is running through the community that suspicion will fall on Masliah’s lab members and even some collaborators. Some are clarifying their collaborations (see comment from Takeshi Iwatsubo, below). Alzforum found that the 132 questioned papers involve more than 550 co-authors. They include junior researchers, scientists at biotech companies, lab technicians, and some of the most prominent names in neurodegeneration research. “It's miserable to think about it,” said Samuel Gandy of Icahn School of Medicine at Mount Sinai in New York City.
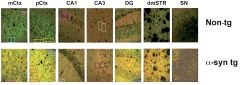
Double Vision? In these brain sections from α-synuclein transgenic and non-transgenic mice, small sections of images appear to be duplicated through “cloning” using photo manipulation software. Colored boxes indicate duplicated regions within the same panel. PubPeer.
Alzforum contacted 39 of Masliah’s former collaborators, asking for comment on the situation and on the nature of their collaborations with him. About half didn’t respond. Others declined, saying their interactions with Masliah’s lab had been minimal—sharing a reagent, for instance, or hiring him as a consultant to analyze samples. Many directed Alzforum to their institutional press offices, which offered no information, or to university lawyers. NIH scientists who worked in Masliah’s division said they had been instructed to refer media requests on the matter to the agency’s communications office.
The few researchers who did speak with Alzforum on the record described Masliah as personable, hard-working, and the rare administrator who was able to run a large government program while continuing to produce his own research. “I always had immense respect for Eliezer,” said Benjamin Wolozin, Boston University. “Not many people are liked by so many others. We have a lot of great scientists but many of us have our personality quirks.”
Wolozin said he thought Masliah was the perfect person to take over the neuroscience portfolio at NIA, which he did in 2016. “His judgment on the field of science was exceptional,” Wolozin said. Nevertheless, “What he did was unacceptable and absolute grounds for dismissal,” Wolozin said.
The NIA said it started its misconduct review process in May 2023 at the request of the Office of Research Integrity, which had received allegations of data manipulation, and started its formal investigation in December 2023. NIA deputy director Amy Kelley is serving as acting director of the neuroscience division.
Neither UCSD nor NIA would answer questions beyond issuing boilerplate statements. “UC San Diego is committed to the highest ethical and legal standards in the conduct of research and we take allegations of research misconduct very seriously,” the university wrote. An NIH spokesperson said the agency “takes allegations of research misconduct seriously. NIH does not discuss whether or not a research misconduct proceeding is taking place and does not comment on NIH proceedings.”
The NIH even declined to confirm which two papers it had identified as containing falsified data, but issued a statement saying they “represent early-stage research conducted in mice on alpha-synuclein, a protein found in neurons. While these studies inform translational research in humans, it is a very small piece of the total research that contributes to the field of neurogenerative diseases.” The two articles, NIH said, have been cited in other papers 18 and 11 times respectively. Additional questions went unanswered.
Two? Or 132?
Commenters on PubPeer and the dossier’s authors flagged issues across at least 132 papers on a variety of topics. The problems range from arguably minor to egregious. Examples include several instances of questionable statistics, such as missing error bars or statistically unlikely data points. In one instance, a photo of a rat, labeled as a mouse, is used to illustrate behavior assays of mice’s purported response to an investigational treatment.

Ratted Out? A data figure on how antibodies against 3R tau reportedly improved mouse behavior contains a photo of a rat, not a mouse. The image is identical to one illustrating Open Field Exploratory Locomotion as an example of rat phenotyping assays on the website of UC Davis’s Rodent Behavior Core (https://tinyurl.com/mrydhw). No researchers from that institution are listed on the paper (Comment #3 on PubPeer page, https://tinyurl.com/bdza27wf.)
The most common concerns involve western blots that contain duplicated bands or multiple images spliced together. One of the most recent papers contains apparently duplicated recording images from a kymograph, which shows movement of lysosomes along axons. Several dozen papers contain histological images that appear in multiple figure panels labeled as coming from different animal lines or from different experimental treatment conditions, or sections of tissue that appear to have been cloned, rotated, deleted or otherwise altered.
Several identical figures appear under different labels in multiple papers published years apart in different journals, including one that is labeled as a rat brain in a 2013 paper and a mouse brain in a 2015 paper. “Normally, when I'm shown these types of images, I have a very hard time figuring out if they are duplicated,” said George Perry, University of Texas at San Antonio. “In this particular case, I did not have a hard time.”
Wolozin has been involved in investigating fraud cases in the past. He said that the variety of alleged problems could be a spectrum ranging from sloppiness to deliberate fraud. “It's going to be difficult for Eliezer to vindicate himself, because in fraud cases, you are presumed guilty until proven innocent,” he said. Researchers accused of misconduct often claim that they lost the original data; if decades have passed since the paper came out, this might be true. “That creates a high bar,” Wolozin said.
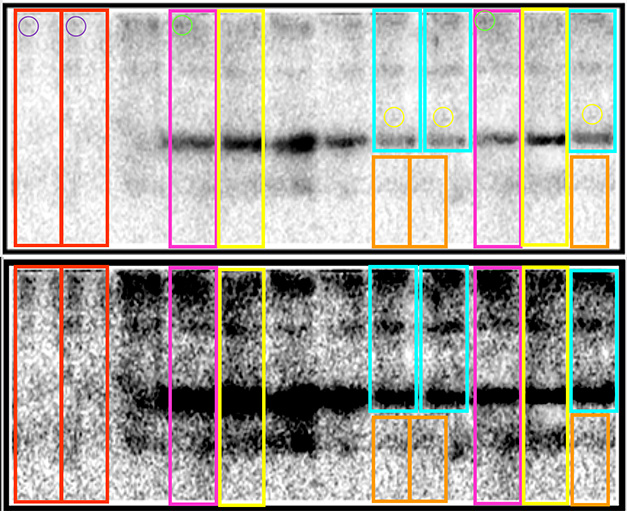
Wild Western? Scores of publications contain western blots that appear to have duplicated lanes or consist of multiple blots spliced together. The dossier’s authors enhanced the contrast to show that boxes show unexpectedly similar lanes. From PubPeer.
Todd Golde, Emory University, Atlanta, was unsurprised that NIH and UCSD have stayed silent. He noted that investigating even a single publication can take months, and that the institutions have dozens of allegations to look into. They may also face legal issues involving companies that have licensed drugs based on Masliah’s data, Golde noted. In some cases, the NIH can force institutions to return grant money that has been spent on fabricated research.
Still, Golde said, slowly performing internal investigations behind closed doors in the face of ample public data suggesting misconduct can look like a cover-up. Speaking off the record, other leading scientists expressed concern that the institutions will slow-walk, minimize, and then attempt to bury an inconvenient issue such as this.
Golde thinks it’s unfortunate that the problems were revealed by a Science magazine news story instead of by a journal or institution. “It's bad for everybody because it's not fair to the field or the investigator,” he said. “There could be honest mistakes; we all make them.”
Duplicating images, as is the case with many of the papers, can be a mistake. Institutions now have the task of determining whether images were intentionally manipulated and, if so, by whom. None of Masliah’s current and former lab technicians Alzforum contacted, some of whom appear on dozens of papers with him, responded, or did so only to request no further contact.
The senior researcher is responsible for data produced in his or her lab. In reality, though, “some of them had to have been complicit,” Wolozin said. “It could be complicit in the sense that they're afraid to take on their boss.” Still, Wolozin suspects there must have been talk. “How could there not be gossip about it? It’s mind-boggling,” he said.
Many scientists are concerned about papers they co-authored with Masliah. Doug Galasko, UCSD, co-authored five papers in the dossier. He said he would categorize their problems as “misconduct.” Galasko said that the clinical core at the Shiley-Marcos ADRC at UCSD, which he directs, mainly worked in parallel with Masliah’s lab to study Lewy body pathology in people with Parkinson’s. Galasko, a neurologist, handled clinical data while Masliah performed pathology on autopsy data, and they combined their findings. “I would have seen the final paper, but wouldn’t have gone through the figures in detail,” he said.
Galasko said he had never heard Masliah’s data questioned publicly. “I don’t have any evidence about motive or how any of these things happened,” he said. “The extent across so many papers does raise serious questions.”
Other professors at UCSD who worked with Masliah declined to answer questions about the respective roles their labs played in the dossier’s publications. Paula Desplats, who appears on 19 of the flagged papers, said she does “not yet have all the details.” Leslie Crews, who collaborated on 23 flagged papers, said she was “unable to comment at this time,” citing work deadlines. Both directed Alzforum to UCSD’s press office, which refused to speak with the press.
Dora Games worked at Athena Neurosciences, South San Francisco, until she retired in 2012. She doubts accusations of deliberate misconduct. “There may very well have been oversights with the volume of work he was doing,” she said, adding that she knew Masliah as a conscientious researcher. “I can't believe that he would intentionally commit fraud or doctor figures to deceive.” Games had not seen the dossier at the time she spoke with Alzforum.
Games worked with Masliah on an α-synuclein-targeting antibody called prasinezumab, which is being developed by Prothena Biosciences for Parkinson’s disease. She said Masliah personally analyzed Athena’s histology samples but was blinded to which were controls and which were treated. “There were a lot of checks and balances in these experiments,” she said. Games appears on six papers listed in the dossier; most of the concerns about them involve western blot manipulation.
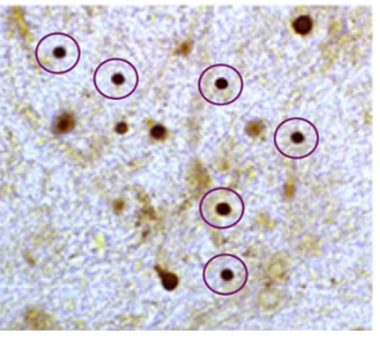
Dot Dot Dot. Neuritic α-synuclein accumulation represented as dark staining. This excerpt of an image panel exemplifies multiple instances of alleged deletion or addition of such foci to Figures 2, 4, 9 of the paper. (From Comment 3 of six comments on PubPeer page https://tinyurl.com/3zjpnzwa.)
Another frequent co-author is Robert Rissman. Of his 41 papers with Masliah, 15 are mentioned in the dossier. Rissman initially agreed to talk with Alzforum, saying he was “in a state of shock” compounded by his considering Masliah to be a mentor and personal friend, but then directed us to USC’s press office. Rissman had worked at UCSD since 2008, and in June 2024 took a position at the University of Southern California, Los Angeles.
USC said they are investigating Rissman’s work with Masliah. “USC takes any allegations of research integrity very seriously,” the university said. “Consistent with federal regulations and USC policies, this review must be kept confidential. As a result, we are unable to provide any further information.”
USC is still conducting an internal review of Berislav Zlokovic, whose research into the role of the blood-brain barrier in AD and stroke drew public scrutiny in 2023 (see Science).
Not all of Masliah’s many co-authors worked with him closely. Markus Mandler, Tridem Bioscience, Vienna, is a co-author on four of the questioned papers. They involved developing immunotherapies against α-synuclein at AFFiRiS. Mandler said that the Austria-based company, founded in 2003 around immunotherapy approaches for Alzheimer’s and Parkinson’s diseases, would ship immunization experiments and samples to UCSD for analysis. “At no time during this collaboration did we have any suspicion that he would be manipulating data, or that he would be using manipulation to support his conclusions,” Mandler said.
Mandler said it would have been difficult to have spotted figure duplication without image-analysis software that was not commonly used 10 years ago. “If you're not thinking about such a thing, you would also not think that this is possible,” he said. Mandler said many other papers from other labs have since validated the papers’ overall conclusion that targeting α-synuclein has potential as an immunotherapy target. “I'm convinced that the main message of the paper stays valid,” he said.
Mandler is no longer affiliated with AFFiRis, and AffiRis is no longer pursuing AD or PD. One of its two programs, AD02, was terminated; the other, PD01, sold to AC Immune.
Martin Citron, UCB, Brussels, said his company had reached similar conclusions regarding a 2023 paper in the dossier on which Masliah was credited as a contractor but not a co-author (Price et al., 2023). The study tested an α-synuclein drug called minzasolmin. UCB licensed it from Neuropore Therapies, a San Diego-based company Masliah co-founded. The question involves one panel of seven in a figure showing mouse hippocampi treated with the drug. UCB says that Masliah was blinded to the animals’ treatment status. The dossier’s authors believe the staining in the control sample appears suspiciously light. Citron and UCB disagree and stand by the paper’s conclusions. “There is nothing that changes, undermines, or otherwise impacts the validity or accuracy of the preclinical results or on the ongoing clinical development program,” a UCB spokesperson said.
Minzasolmin is in Phase 2 trials, with results expected later this year. Citron said that the clinical data outweighs preclinical mouse data, and that the latter has been replicated elsewhere. “It would be very difficult for a single investigator to misdirect an entire field,” he told Alzforum, but added that the allegations are depressing. “At the end of the day this helps nobody,” Citron said.
“The most unforgivable part is if there is scientific misconduct and data manipulation supporting therapeutics that are moving into clinical trials. That’s a huge problem,” Golde said. “People would be put at unnecessary risk for something for which there isn’t scientific evidence.” The NIH paused a trial of an investigational stroke drug by USC’s Zlokovic; no such action has been taken with Masliah’s work (Los Angeles Times).
Eight of the dossier’s papers concern cerebrolysin, a treated peptide extract from cattle brain that is being administered for cerebrovascular conditions in European countries. Cerebrolysin is not approved in the U.S. For example, Rockenstein et al., 2015, has 13 flags, with image overlap animations (PubPeer). For another example, see image below.
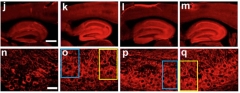
All in One? According to PubPeer Comment 2 to this paper, panels o, p, q appear to come from the same hippocampus. Panels o and q are published to represent different mouse lines treated with cerebrolysin; p purports to show a vehicle-treated mouse. Colored boxes outline the overlap; comment 3 links to animation on PubPeer page.
According to the dossier, four of Masliah’s papers on preclinical models that supported the development of prasinezumab appear to contain manipulated images. The drug is in Phase 2. One study reported no efficacy after one year on prasinezumab, and serious adverse events in 7 percent of treated patients; however, subgroup analyses and a long-term extension hint at slower decline in certain groups after several years of treatment (Pagano et al., 2022). Researchers caution that it’s too soon to associate the drug’s performance with potential manipulations in preclinical data. “We really need to see discussion about how these results have been replicated over and over again in different ways by independent people,” Games said.
“Clinical trials fail for many reasons,” Wolozin said, including different mechanisms driving the mouse phenotype and human disease, or not enough antibodies getting into the brain. “These are things that can only be sorted out, unfortunately, in clinical trials.”
Coming: A Wave of Corrections and Retractions?
Journals in which the questioned papers are published are now investigating the figures. Alzforum identified 13 corrections to Masliah’s papers currently in PubMed. Most are made in response to older flags on PubPeer that precede by several years the current investigation by Science; a few are unrelated to Masliah's lab.
One paper has been retracted (Iba et al., 2022, PubPeer). The journal editors' retraction notice, posted October 16, reads, in part, "The authors have stated that incorrect images were included in the article by mistake and provided the raw data for validation. However, further checks by the publisher found highly similar images between the raw data provided for different groups or experiments."
One recent correction concerns a 2022 paper in NPJ Vaccines that tested an α-synuclein vaccine called PV-1950D in mice (Kim et al., 2023). In a comment on PubPeer, the paper’s senior author, Michael Agadjanyan, Institute for Molecular Medicine, Huntington Beach, California, expressed surprise that he had not been made aware of the correction to four panels in a figure that contained duplicated images of hippocampi. Nor was the correction complete: The dossier found one other panel in the figure was identical to one in a separate figure but had been rotated 180 degrees.
An email apparently sent by Masliah to NPJ Vaccines attributed the problem to “having multiple panels opened during the assembling of the multi-panel figure and accidentally not using the correct panel of hippocampus treated with PV-1950D in the erratum that was submitted in 2023.” Masliah wrote he was going to send the journal a fully corrected figure. Agadjanyan, in his indignation, shared the email with Alzforum. Our requests to confirm its authenticity with Masliah received no response. The journal’s managing editor declined to confirm the correspondence, but did reply to say that, on October 15, 2024, the journal added a note of concern to the paper (Kim et al., 2024).
“I am shocked and deeply troubled by the potential implications of Dr. Masliah's scientific misconduct,” Agadjanyan told Alzforum, adding that he is actively looking into what this might mean for his prior collaboration. Among the dozens of implicated papers, multiple examples exist where corrections appear to have been partial, or irregularities were subsequently flagged in the corrections themselves (e.g. Dhungel et al. 2023 on PubPeer).
The latest correction to one of Masliah’s papers, which is not in the dossier or PubPeer, was posted October 3, 2024. It concerned a histological image of a hippocampus that had been duplicated across two figures in a paper in EMBO Reports (Maeda et al., 2016). Its senior author, Lennart Mucke, University of California, San Francisco, appears on 47 papers with Masliah, seven of which are in the dossier. “It would be inappropriate for me to comment on this issue until I have developed a better understanding of all details involved,” Mucke wrote to Alzforum.
One journal—PLoS ONE—has thus far issued an expression of concern, a step typically taken before a retraction (Duka et al., 2013). The paper, about tau phosphorylation, on which Masliah was a co-author, contained suspiciously similar western blot bands, among other possible manipulations. “The authors did not respond to the concerns that were raised regarding these figures, and no underlying data was provided for editorial review,” the journal editors wrote. The paper’s corresponding author, Anita Sidhu, Georgetown University, Washington, D.C., did not respond to interview requests.
PLoS ONE posted its notice on December 5, 2023, the same month in which the NIH launched its investigation. The agency would not confirm whether this paper was one of the two it had identified as containing falsified data.
Alzforum asked the NIH why, given the dozens of people whose published work may now face correction or retraction, it was not releasing more information about its investigation. The agency did not respond.
“One should not condemn anybody who was working with Masliah just because [they were] working with him,” Mandler said. “Regardless of who is at fault, this a warning for the field that you really need to always make very thorough analysis and make sure everything is correct,” he added.
Perry blamed pressure to publish exciting data in high-impact journals, rather than replicating existing data. The dossier flags three papers in the Journal of Alzheimer’s Disease, of which Perry is the senior editor. He said he is taking them seriously but is unsure how his journal could have caught them earlier. “We're responding to, rather than looking for, the problem. I think that's what most journals are doing,” he said. “To have image-analysis people on board to be able to do this, and the software, requires quite a commitment.”
Gandy agrees that duplicate images in papers published years apart would be impossible to spot “unless there's AI control of every image that enters the literature and compares it against every other image in the literature.” That said, the image-analysis software used by PubPeer sleuths surely could be deployed by major journals to prevent embarrassment later.
Wolozin was surprised that Masliah’s publications contain so many duplicated images. “It seems like he continued that up to now through the digital revolution,” Wolozin said. “I'm shocked that he wouldn't have thought he would be caught at some point.”
Five of the approximately 130 papers were covered on Alzforum when they came out. One has two flags, for an image and a western blot area (Masliah et al., 2001, PubPeer). One has two video animations for seven flags about western blots and microscopy images (Masliah et al., 2005, PubPeer). One has problematic western blots, a fourth has overlapping immunohistochemistry images labeled as coming from different Alzheimer’s patients as well as potentially cloned sections in electron micrographs (Rockenstein et al., 2007, PubPeer; Pickford et al., 2008, PubPeer). A fifth has a troublesome western blot (Dziewczapolski et al., 2009, PubPeer).
Overall, Masliah’s case of alleged malpractice is the most extensive but not the only recent one in neurodegeneration research. Besides USC’s Zlokovic, Marc Tessier-Lavigne lost the presidency of Stanford over irregularities in his lab there and previously at Genentech (e.g., May 2014 news). Nobel laureate Tom Südhof of Stanford is defending his career with a dedicated section of his lab website detailing changes in lab organization and transparency (Südhof Laboratory Integrity Initiatives). Prion researcher Adriano Aguzzi of the University of Zurich is dealing with public accusations (Blick news story).
After years of controversy, Cassava, the company behind simufilam, in September 2024 was fined $40 million by the Securities and Exchange Commission and its husband-and-wife leadership team let go. The University of Minnesota’s Sylvain Lesne has vanished from public view after allegations ensnared a 2006 Nature paper that had made an initial splash but was never replicated by, nor influenced, other Alzheimer’s research labs (Jul 2022 news).
The NIH is trying to address the broader issue of reproducibility, e. g., with an initiative to support independent replication that includes grants (e.g. this one). What else can scientists do? To share ideas, contact us.—Sara Reardon and Gabrielle Strobel
Sara Reardon is a freelance writer in Bozeman, Montana.
References
Basic page Citations
Therapeutics Citations
News Citations
- Reports Weaken Death Receptor Link to Alzheimer's Disease
- Sylvain Lesné, Who Found Aβ*56, Accused of Image Manipulation
Grants Citations
Paper Citations
- Price DL, Khan A, Angers R, Cardenas A, Prato MK, Bani M, Bonhaus DW, Citron M, Biere AL. In vivo effects of the alpha-synuclein misfolding inhibitor minzasolmin supports clinical development in Parkinson's disease. NPJ Parkinsons Dis. 2023 Jul 17;9(1):114. PubMed.
- Rockenstein E, Ubhi K, Mante M, Florio J, Adame A, Winter S, Brandstaetter H, Meier D, Masliah E. Neuroprotective effects of Cerebrolysin in triple repeat Tau transgenic model of Pick's disease and fronto-temporal tauopathies. BMC Neurosci. 2015 Nov 26;16:85. PubMed.
- Pagano G, Taylor KI, Anzures-Cabrera J, Marchesi M, Simuni T, Marek K, Postuma RB, Pavese N, Stocchi F, Azulay JP, Mollenhauer B, López-Manzanares L, Russell DS, Boyd JT, Nicholas AP, Luquin MR, Hauser RA, Gasser T, Poewe W, Ricci B, Boulay A, Vogt A, Boess FG, Dukart J, D'Urso G, Finch R, Zanigni S, Monnet A, Pross N, Hahn A, Svoboda H, Britschgi M, Lipsmeier F, Volkova-Volkmar E, Lindemann M, Dziadek S, Holiga Š, Rukina D, Kustermann T, Kerchner GA, Fontoura P, Umbricht D, Doody R, Nikolcheva T, Bonni A, PASADENA Investigators and Prasinezumab Study Group. Trial of Prasinezumab in Early-Stage Parkinson's Disease. N Engl J Med. 2022 Aug 4;387(5):421-432. PubMed.
- Iba M, McDevitt RA, Kim C, Roy R, Sarantopoulou D, Tommer E, Siegars B, Sallin M, Kwon S, Sen JM, Sen R, Masliah E. Aging exacerbates the brain inflammatory micro-environment contributing to α-synuclein pathology and functional deficits in a mouse model of DLB/PD. Mol Neurodegener. 2022 Sep 5;17(1):60. PubMed. RETRACTED
- Kim C, Hovakimyan A, Zagorski K, Antonyan T, Petrushina I, Davtyan H, Chailyan G, Hasselmann J, Iba M, Adame A, Rockenstein E, Szabo M, Blurton-Jones M, Cribbs DH, Ghochikyan A, Masliah E, Agadjanyan MG. Author Correction: Efficacy and immunogenicity of MultiTEP-based DNA vaccines targeting human α-synuclein: prelude for IND enabling studies. NPJ Vaccines. 2023 Jul 17;8(1):105. PubMed. Corrected paper.
- Maeda S, Djukic B, Taneja P, Yu GQ, Lo I, Davis A, Craft R, Guo W, Wang X, Kim D, Ponnusamy R, Gill TM, Masliah E, Mucke L. Expression of A152T human tau causes age-dependent neuronal dysfunction and loss in transgenic mice. EMBO Rep. 2016 Apr;17(4):530-51. Epub 2016 Mar 1 PubMed. Correction.
- Duka V, Lee JH, Credle J, Wills J, Oaks A, Smolinsky C, Shah K, Mash DC, Masliah E, Sidhu A. Identification of the sites of tau hyperphosphorylation and activation of tau kinases in synucleinopathies and Alzheimer's diseases. PLoS One. 2013;8(9):e75025. PubMed. Expression of concern.
- Masliah E, Rockenstein E, Veinbergs I, Sagara Y, Mallory M, Hashimoto M, Mucke L. beta-amyloid peptides enhance alpha-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer's disease and Parkinson's disease. Proc Natl Acad Sci U S A. 2001 Oct 9;98(21):12245-50. Epub 2001 Sep 25 PubMed.
- Masliah E, Rockenstein E, Adame A, Alford M, Crews L, Hashimoto M, Seubert P, Lee M, Goldstein J, Chilcote T, Games D, Schenk D. Effects of alpha-synuclein immunization in a mouse model of Parkinson's disease. Neuron. 2005 Jun 16;46(6):857-68. PubMed.
- Rockenstein E, Torrance M, Adame A, Mante M, Bar-On P, Rose JB, Crews L, Masliah E. Neuroprotective effects of regulators of the glycogen synthase kinase-3beta signaling pathway in a transgenic model of Alzheimer's disease are associated with reduced amyloid precursor protein phosphorylation. J Neurosci. 2007 Feb 21;27(8):1981-91. PubMed.
- Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, Small S, Spencer B, Rockenstein E, Levine B, Wyss-Coray T. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Invest. 2008 Jun;118(6):2190-9. PubMed.
- Dziewczapolski G, Glogowski CM, Masliah E, Heinemann SF. Deletion of the alpha 7 nicotinic acetylcholine receptor gene improves cognitive deficits and synaptic pathology in a mouse model of Alzheimer's disease. J Neurosci. 2009 Jul 8;29(27):8805-15. PubMed.
Other Citations
External Citations
- investigative story
- PubPeer website
- PubPeer
- 2024 NIH Alzheimer’s Research Summit
- statement
- PubPeer
- kymograph
- PubPeer
- Science
- Neuropore Therapies
- Los Angeles Times
- PubPeer
- PubPeer
- PubPeer
- comment on PubPeer
- Kim et al., 2024
- PubPeer
- PubPeer
- PubPeer
- PubPeer
- PubPeer
- PubPeer
- Südhof Laboratory Integrity Initiatives
- Blick news story
- initiative
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
University of Tokyo
PubMed lists four papers co-authored by Dr. Masliah and myself.
1. and 2. are review papers by multiple experts.
3. is one of our most important publications, showing Ser129 phosphorylation of α-synuclein, which is a well-replicated finding in α-synuclein pathobiology. The reason I invited Dr. Masliah to participate in this paper was because one of the reviewers asked that we add phospho-synuclein immunostaining in the brains of transgenic mice, which at that time had only been generated by two groups, Dr. Jie Shen’s and Dr. Masliah’s. I asked Dr. Masliah to share some paraffin-embedded sections of his transgenic mouse brains that had been published in his 2000 Science paper.
Fujiwara et al., 2002, got attention on PubPeer in September 2024, with anonymous comments saying that numbers and error bars were missing from a standard curve. I don't think this is a problem, and another anonymous reviewer made the following comment:
"I'm not involved in this paper, not an author or anything related but … this is a standard curve so error bars and N aren't statistically relevant. Sometimes people put indications of uncertainty on a standard curve to show technical performance such as CV or range between replicates, but these are not independent samples, typically dilutions from a master reagent (peptide or protein in this case). It isn't technically necessary to even run replicates in a standard curve—possibly unwise if the measurements have high intra-sample variability but not wrong and definitely not fraud.
"And the citations? Yes, it's highly cited because everyone who has tried to replicate the main finding, that this protein is phosphorylated in the Parkinson's disease brain, has been able to do so."
Therefore I believe this paper is sound and outside any concerns related to the possible misconduct by Dr. Masliah.
4. is senior-authored by Dr. Masliah. The reason why I was invited may have been to share the antibody generated in the above Nat Cell Biol paper. It is on PubPeer, with multiple comments and a video animation regarding image overlap (PubPeer). My lab did not conduct experiments, nor prepare images, for this paper.
References:
Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, Castellani RJ, Crain BJ, Davies P, Del Tredici K, Duyckaerts C, Frosch MP, Haroutunian V, Hof PR, Hulette CM, Hyman BT, Iwatsubo T, Jellinger KA, Jicha GA, Kövari E, Kukull WA, Leverenz JB, Love S, Mackenzie IR, Mann DM, Masliah E, McKee AC, Montine TJ, Morris JC, Schneider JA, Sonnen JA, Thal DR, Trojanowski JQ, Troncoso JC, Wisniewski T, Woltjer RL, Beach TG. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012 May;71(5):362-81. PubMed.
Lippa CF, Duda JE, Grossman M, Hurtig HI, Aarsland D, Boeve BF, Brooks DJ, Dickson DW, Dubois B, Emre M, Fahn S, Farmer JM, Galasko D, Galvin JE, Goetz CG, Growdon JH, Gwinn-Hardy KA, Hardy J, Heutink P, Iwatsubo T, Kosaka K, Lee VM, Leverenz JB, Masliah E, McKeith IG, Nussbaum RL, Olanow CW, Ravina BM, Singleton AB, Tanner CM, Trojanowski JQ, Wszolek ZK, DLB/PDD Working Group. DLB and PDD boundary issues: diagnosis, treatment, molecular pathology, and biomarkers. Neurology. 2007 Mar 13;68(11):812-9. PubMed.
Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002 Feb;4(2):160-4. PubMed.
Shults CW, Rockenstein E, Crews L, Adame A, Mante M, Larrea G, Hashimoto M, Song D, Iwatsubo T, Tsuboi K, Masliah E. Neurological and neurodegenerative alterations in a transgenic mouse model expressing human alpha-synuclein under oligodendrocyte promoter: implications for multiple system atrophy. J Neurosci. 2005 Nov 16;25(46):10689-99. PubMed.
Vice President, The Institute for Molecular Medicine
I would like to clarify exactly how I responded to the Alzforum reporter's email requesting a comment.
"Thank you for your email. I returned from vacation on Monday and didn't have much additional insight into this now. I have not been given a copy of the dossier and only just heard about this troubling news two days ago. Nevertheless, I am shocked and deeply troubled by the potential implications of Dr. Masliah's scientific misconduct. I am actively looking into the potential implications of this on our prior collaboration with Eliezer Masliah. As soon as I have more information, I will be glad to follow up with you.”
I further informed Alzforum and Pubpeer that Dr. Masliah sent us and Dr. Barret, the editor in chief of NPJ Vaccines, all the correct images and data for Figure 7. I expressed hope that NPJ Vaccines would correct Figure 7 soon, after which I would provide more information to Alzforum. It seems that the journal is currently investigating the materials submitted by Dr. Masliah, and I will update all my collaborators from various universities, NIH, and the scientific community through Alzforum and Pubpeer, as soon as I receive the journal's response.
Regarding the Fig. 7 problem, it might be an honest mistake in one of the 384 representative images provided solely for illustration purposes, which had no impact on the study results and conclusions.
Harvard Medical School
Thank you Alzforum and Science Magazine for focusing on this issue! Addressing this openly is very important. Silence can be a form of complicity. Several people are now trying to parse details and find relevance versus specific fabrications. That can miss an overarching truth around the general intent of the body of works. This intent carries a more destructive capacity than any specific altered data point discovered so far. This fraud is therefore likely greater than has been, and can be, discovered.
The sciences rely almost entirely on observations of facts, and the best inferences of what these facts mean in terms of knowledge generation. Therefore, when such facts are adulterated, the outcome can only be chaos and misdirection. As in the saying “It’s much easier to tell a lie when it’s hard to tell the truth,” the fictionalization of data becomes the ultimate enemy of science. Human behavior is no different today than at any other time in history. When some scientists systematically betray trust, everyone suffers the consequences.
This is the case also in the latest story of Masliah’s group’s work in pathology for a number of neurodegenerative diseases, such as Alzheimer’s and Parkinson’s. There are probably more misleading data from this team that have not yet been discovered. For example, some original papers that are now listed on the PubPeer site have confused us for years, and we believe that the active α-synuclein “spread” theory for Parkinson’s disease also has been fed by data that is likely to be false or misinterpreted by this scientific group and followers (e.g. Desplats et al., 2009).
This is not trivial, since it still drives drug development in the clinic and by companies. We all know that oversimplification and fabrication of data can serve theories of indoctrinated scientists and industry, and such circles powerfully protect their beliefs. Any excellent and unassuming scientist (and co-authors), can be betrayed by highly effective liars, including when they became the head of NIA's Neuroscience Division. Such falsehoods can control many decisions in science/key leaders/academia/industry circles, so that real progress will slow or stop, and patients continue to suffer because of ineffective drugs generated by such false and misleading knowledge.
References:
Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009 Aug 4;106(31):13010-5. PubMed.
Make a Comment
To make a comment you must login or register.