Nanobubbles: Potent Potion, or Just Effervescence?
Quick Links
The tinier the bubbles in a glass of champagne, the quicker the booze goes to your head, some say. At the Society for Neuroscience meeting held November 15 to 19 in Washington, D.C., scientists presented surprising findings that suggest the smallest bubbles of all—nanobubbles—may boost cognition and even fight Alzheimer’s disease. The bulk of the data came from Revalesio, a small company based in Tacoma, Washington, and its collaborators.
Nanobubbles are not found in Dom Perignon. In Revalesio’s formulation, they are charged microscopic bubbles filled with oxygen, not carbon dioxide. Researchers have reported that these tiny spheres achieve all manner of cellular heroics, including soothing neuroinflammation, blocking tau phosphorylation, saving neurons from the brink of death, boosting synaptic plasticity, and even rescuing memory deficits in AD mouse models. Clinical trials are already underway to treat multiple sclerosis and asthma, with one for AD on the horizon. Research into the bubbles and their potential applications has taken off recently: A conference devoted to nanobubbles took place in Shanghai last October.
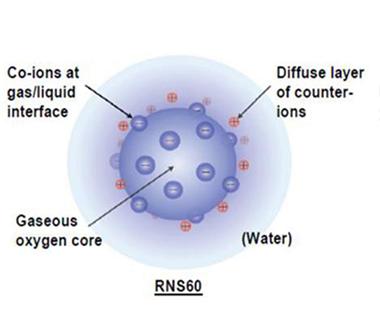
Powerful Packages.
Charged ions line the surface of nanobubbles filled with oxygen in this depiction of the structure of RNS60. [Image courtesy of Roy et al., PLOS One, 2014.]
The way these wee bubbles work their magic remains a mystery, and many are still skeptical. However, researchers reported that most of their effects can be explained by activation of phosphatidylinositol-3-kinase (PI3K) and/or a boost in mitochondrial function.
Nanobubbles were discovered serendipitously in the late 1990s by Tony Wood, a then-retired Texas Instruments scientist. In his garage, he tinkered with ways to mix gases and liquids more efficiently. When his mixtures retained dissolved oxygen for longer than expected, Wood discovered that nanobubbles were the reason. He then launched a company aiming to use the bubble formulation for agricultural applications, including improving plants’ resistance to disease. Revalesio later bought Wood’s company and further honed the bubble-creation technique. Currently its researchers generate RNS60, Revalesio's oxygen-filled nanobubble formulation, via a process involving something called Taylor-Couette-Poiseuille (TCP) flow. This is a mixing technique in which a saline solution is whisked rapidly under pressurized oxygen. Wood himself was diagnosed with amyotrophic lateral sclerosis in 2011. In 2012, following Revalesio’s completion of a Phase I safety study in healthy volunteers, Wood started receiving infusions of the bubbles under an FDA compassionate-use protocol. By the start of his treatment, Wood’s condition had already progressed to the point where he relied upon a ventilator, but he has since remained stable throughout nearly three years of treatment. Revalesio cannot conclude whether the plateauing of Wood’s disease is related to the nanobubble infusions.
Revalesio aims to treat people with inflammatory disorders, including multiple sclerosis and asthma, with RNS60. The plans are based on preclinical data suggesting that the bubbles calm microglial responses and boost regulatory T cells, which douse the overeager responses of other T cells that wreak havoc in autoimmune disease (see Khasnavis et al., 2012, and Mondal et al., 2012). Revalesio’s scientists later found that in addition to their effects on inflammation, the bubbles directly protected neurons. At SfN, Avik Roy, a researcher from Kalipada Pahan’s laboratory at Rush University in Chicago, presented a sampling of the data implicating the bubbles in neural regeneration.
Substance Behind the Bubbles
Roy and colleagues chipped away at the mechanism behind the phenomenon. They found that treatment with RNS60 boosted the growth, length, and density of dendritic spines on cultured hippocampal neurons. The treatment also upped the expression of NMDA and AMPA receptor subunits NR2A and GluR1, resulting in an increase in calcium intracellular signaling. In a gene-expression analysis, treatment with RNS60 turned on 62 of 84 genes associated with synaptic plasticity, including CREB; immediate early genes such as ZIF-268, Arc, and c-Fos; synapse-associated genes including PSD-95; and neurotrophic factors such as BDNF. Conversely, RNS60 quenched some genes known to dampen synaptic plasticity. PI3K inhibitors blocked all the gains in synaptic plasticity triggered by the bubbles, suggesting this signaling pathway was central to their broad actions.
The researchers then tried out RNS60 in 5xFAD mice, an aggressive AD model. Compared with those in normal mice, hippocampal neurons in 5xFAD mice had lower levels of key synaptic proteins, and reduced calcium signaling in response to stimulation with neurotransmitters, but two months of daily intraperitoneal injections of RNS60 reversed those effects. The researchers reported these data last July in PLoS One (Roy et al., 2014).
In the August 2 issue of the same journal, the researchers reported that RNS60 treatment rescued deficits in learning and spatial memory in 5xFAD mice (see Modi et al., 2014). RNS60 protected hippocampal neurons in these mice from apoptotic cell death, and shielded cultured neurons from death inflicted by Aβ. The bubbles also prevented hyperphosphorylation of tau in vivo in hippocampal neurons from 5xFAD mice, and downregulated the activity of GSK3b, a kinase implicated in phosphorylating tau, in cultured neurons. As with the synaptic plasticity boost, RNS60’s sway over neuronal death, tau phosphorylation, and GSK3b activity depended on the activation of PI3K, which was temporarily buoyed by the bubbles.
Revalesio’s recent findings mesh with those from Rodolfo Llinás’s lab at New York University in New York. An electrophysiologist whose bread and butter for 50 years has been the squid giant synapse, Llinás told Alzforum that he was at first intrigued by (and highly skeptical of) Revalesio’s findings. He decided to test the bubbles for himself. He reported that RNS60 improved synaptic transmission in squid neurons by mobilizing more vesicles to the synapse. Mitochondria in the treated neurons produced more ATP than usual. Llinás and colleagues reported that blocking ATP synthesis abolished all of RNS60’s positive effects (see Choi et al., 2014). Llinás’s lab has since found that RNS60 not only revs up ATP production, but enlarges mitochondria, according to a poster he presented at SfN. Rather than altering the kinetics of synaptic transmission, which could prove harmful, Llinás said that RNS60 simply injects more fuel into the energetically costly process. “I see it as a sort of ‘life optimizer’ rather than a drug,” Llinás said. “It makes everything run as efficiently as possible.” Llinás and colleagues also have a paper in press that reports similar findings in Xenopus giant oocytes.
How does it work? Llinás hypothesizes that the bubbles boost ATP production by delivering oxygen to the mitochondria. Normally, too much oxygen can be harmful, but Llinás said that he and his NYU colleagues Maxim Ivannikov and Mutsuyuki Sugimori have evidence that the bubbles cross the outer cell membrane as well as the mitochondrial membrane, then release the oxygen once inside the powerhouse organelle’s interior. In this way, the bubbles deliver their cargo without creating toxic free radicals in the cell, Llinás said. He thinks a majority of the effects both his lab and Revalesio’s researchers have reported stem from the extra ATP production triggered by this fuel injection.
Revalesio researchers are currently looking into the role of enhanced mitochondrial function in RNS60’s effects. However, for now they say their evidence points to the activation of PI3K as the major event that underlies most of the bubbles’ benefits. “Although it seems like there are various effects of RNS60, it actually has come down so far to a single signaling pathway: PI3K/Akt,” said Supurna Ghosh, Revalesio’s director of neurology research. “All of the effects we have seen so far in T cells, glial cells, or neurons can be abrogated by blocking that pathway.” How would the bubbles activate this pathway? “That’s the key question, and we really do not have an answer for it yet,” Ghosh said. PI3K can be activated from outside the cell via receptors on the cytoplasmic membrane, or from within the cell via other signaling molecules. “It’s possible that the bubbles enter the cell and activate it from inside, or possible that bubbles interfere with other proteins on the cell membrane, in turn activating PI3K,” she said.
Whether the bubbles’ benefits are derived from their physical interactions with proteins or from the way they deliver oxygen to mitochondria, it seems clear from the studies that both the bubble and the oxygen are key, Ghosh said. Control experiments with oxygenated fluids, or bubbles without oxygen, did not produce any of RNS60’s effects. This may dash the hopes of people hoping to glean some of the nanobubbles’ benefits by taking a few hits of oxygen at an oxygen bar (the establishments are growing in popularity and some claim to provide health benefits). Forcing oxygen into tissues by spending time in a hyperbaric chamber may also sound similar, however Watson said he thinks the bubbles’ structure is the key to their effects.
Regardless of exactly how the bubbles work, it seems likely that they are somehow delivered to the CNS, judging by experiments in mice, Ghosh said. Another recent study by the group reported that three hours after of being injected intraperitoneally, nanobubbles activated PI3K in the substantia nigra (see Khasnavis et al., 2014). Currently, there is no way to track the teeny bubbles in vivo, but Richard Watson, Revalesio’s chief scientific officer, said researchers are working on ways to do so by detecting gaseous oxygen. He estimates that the bubbles stick around in the body for about 18 hours before disintegrating.
RNS60 has so far proven safe in Phase 1 trials when injected intravenously or inhaled by healthy people or people with asthma (see clinical trials.gov; clinical trials,gov), and Phase 2 trials for both multiple sclerosis and asthma are about to begin, Watson said (see clinicaltrials.gov). Revalesio is currently devising inclusion criteria for its AD trial, which is expected to begin next year. It plans to enroll people with MCI or in the early stages of AD, and will use a combination of imaging and cognitive measures to monitor effects of RNS60.
At SfN, Watson was enthused by a presentation by Cynthia Lemere of Brigham and Women’s Hospital, Boston, which showcased GE180, a new PET imaging tracer for neuroinflammation (see Part 4 of this series). Watson hopes something like this could be a useful outcome measure in the RNS60 trial.
Lemere and Revalesio are hatching a plan to collaborate. Lemere plans to test RNS60 in AD mouse models, and use the GE180 tracer to measure its effects on neuroinflammation, she told Alzforum. Like most scientists, Lemere had her reservations about the bubbles at first. “It sounds far-fetched and bizarre, but the data looks really good and they’re doing rigorous science,” she said. While Lemere doesn’t believe that any one treatment will be a cure for AD, she said RNS60 could have potential, especially in combination with other therapies. “The fact that this has such broad application is really interesting,” she said. “I believe inflammation plays a big role in disease progression, so if you can quell the inflammation early in that process, you might be able to slow down neurodegeneration.”—Jessica Shugart
References
Research Models Citations
News Citations
Paper Citations
- Khasnavis S, Jana A, Roy A, Mazumder M, Bhushan B, Wood T, Ghosh S, Watson R, Pahan K. Suppression of Nuclear Factor-κB Activation and Inflammation in Microglia by Physically Modified Saline. J Biol Chem. 2012 Aug 24;287(35):29529-42. PubMed.
- Mondal S, Martinson JA, Ghosh S, Watson R, Pahan K. Protection of Tregs, suppression of Th1 and Th17 cells, and amelioration of experimental allergic encephalomyelitis by a physically-modified saline. PLoS One. 2012;7(12):e51869. Epub 2012 Dec 20 PubMed.
- Roy A, Modi KK, Khasnavis S, Ghosh S, Watson R, Pahan K. Enhancement of morphological plasticity in hippocampal neurons by a physically modified saline via phosphatidylinositol-3 kinase. PLoS One. 2014;9(7):e101883. Epub 2014 Jul 9 PubMed.
- Modi KK, Jana A, Ghosh S, Watson R, Pahan K. A physically-modified saline suppresses neuronal apoptosis, attenuates tau phosphorylation and protects memory in an animal model of Alzheimer's disease. PLoS One. 2014;9(8):e103606. Epub 2014 Aug 4 PubMed.
- Choi S, Yu E, Rabello G, Merlo S, Zemmar A, Walton KD, Moreno H, Moreira JE, Sugimori M, Llinás RR. Enhanced synaptic transmission at the squid giant synapse by artificial seawater based on physically modified saline. Front Synaptic Neurosci. 2014;6:2. Epub 2014 Feb 12 PubMed.
- Khasnavis S, Roy A, Ghosh S, Watson R, Pahan K. Protection of dopaminergic neurons in a mouse model of Parkinson's disease by a physically-modified saline containing charge-stabilized nanobubbles. J Neuroimmune Pharmacol. 2014 Mar;9(2):218-32. PubMed.
External Citations
Further Reading
No Available Further Reading
Primary Papers
- Modi KK, Jana A, Ghosh S, Watson R, Pahan K. A physically-modified saline suppresses neuronal apoptosis, attenuates tau phosphorylation and protects memory in an animal model of Alzheimer's disease. PLoS One. 2014;9(8):e103606. Epub 2014 Aug 4 PubMed.
- Roy A, Modi KK, Khasnavis S, Ghosh S, Watson R, Pahan K. Enhancement of morphological plasticity in hippocampal neurons by a physically modified saline via phosphatidylinositol-3 kinase. PLoS One. 2014;9(7):e101883. Epub 2014 Jul 9 PubMed.
- Khasnavis S, Roy A, Ghosh S, Watson R, Pahan K. Protection of dopaminergic neurons in a mouse model of Parkinson's disease by a physically-modified saline containing charge-stabilized nanobubbles. J Neuroimmune Pharmacol. 2014 Mar;9(2):218-32. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.