Paper Alert: Aducanumab Phase 1b Study Published
Quick Links
Scientists at Biogen have formally published the results of a clinical trial that dazzled the Alzheimer's disease field last year: Aducanumab, a monoclonal antibody against Aβ, wiped out a hefty proportion of plaques in the brains of people with AD. The findings, reported in the August 31 Nature, also hint at a functional benefit. On two tests of cognition, people on the drug declined more slowly and in a roughly dose-dependent manner. Incidences of brain-imaging abnormalities called ARIA-E, indicative of fluid build-up in the brain, also cropped up dose-dependently, but these abnormalities were transient. Alfred Sandrock of Biogen in Cambridge, Massachusetts, and Roger Nitsch of Neurimmune in Zurich co-led the trial. Some researchers lamented the overinterpretation of underpowered cognitive data, but applauded the drug’s apparent plaque-fighting powers. The field now awaits the results of two ongoing Phase 3 trials, powered to conclusively tease out any clinical benefits.
Dennis Selkoe of Brigham and Women’s Hospital in Boston said that the peer-reviewed data buoyed the optimism he first felt after seeing the top-line results presented last year at the AD/PD conference in Nice (see Mar 2015 conference news). “As a clinician who sees people with AD, this is the kind of data that would encourage me to enroll a patient in the aducanumab trial,” Selkoe said. “I think it is highly likely that the Phase 3 trials will have a clinical benefit.”
Colin Masters of the University of Melbourne in Australia agreed that the published data were strong. “The numbers are very compelling and provide a great impetus for the field to really understand the molecular basis by which aducanumab promotes the clearance of Aβ from the brain,” he commented.
Aducanumab recognizes the N-terminus of the Aβ peptide. Selected from a library of antibodies from adults in their 70s with no signs of amyloid accumulation (see Apr 2011 conference news), the antibody latches onto oligomeric and fibrillar forms of Aβ, but leaves monomers alone. Rodent studies, reported in the current paper as well as previously at conferences, indicated that the antibody bound to parenchymal Aβ plaques, and recruited microglia to encircle them. The antibody triggered the clearance of plaques of all sizes (see Jan 2013 conference news).
The antibody appeared safe in a Phase 1 clinical trial (see Apr 2013 conference news). Results from the subsequent Phase 1b trial made a huge splash in Nice last year when presented by Jeff Sevigny, the first author of the Nature paper, who has since left Biogen for Roche. That trial enrolled 165 patients with prodromal or mild AD who had florbetapir PET evidence of Aβ accumulation. At 33 centers in the United States, participants were randomized to receive roughly monthly infusions of placebo, or 1, 3, 6, or 10 mg/kg doses of aducanumab for one year. They underwent cognitive tests as well as florbetapir-PET scans at baseline, six months, and 12 months. At the two later time points, the researchers observed a dramatic and dose-dependent reduction of Aβ deposits. Compared to the placebo group, in which the florbetapir standardized uptake value ratio (SUVR) hovered around 1.44, the average uptake in the 10 mg/kg group dropped to 1.16, within spitting distance of the 1.10 cut-off that divides amyloid positive and negative scans. Plaque load diminished in people with both prodromal and mild AD and regardless of ApoE4 carrier status.
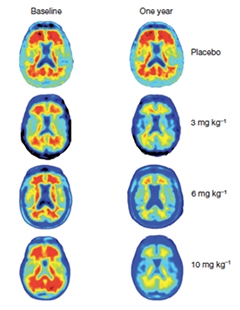
Aβ Reduction.
After one year of treatment with aducanumab, Aβ plaques were substantially removed from the brains of people with AD. [Image courtesy of Sevigny et al., Nature 2016.]
Though the researchers emphasized that the study was too small to definitively prove cognitive effects, they nonetheless reported that people in the treatment groups declined less in their performance on the Clinical Dementia Rating-Sum of Boxes (CDR-SB) between six months and one year than those given placebo. Slowing of decline occurred dose-dependently, and people in the 10 mg/kg group had similar scores on the test at both time points. Results were not perfectly dose-dependent on the Mini Mental State Exam (MMSE), in which decline in the 3 mg/kg and 10 mg/kg groups was significantly less than placebo, while the 1 mg/kg and 6 mg/kg groups only trended toward protection. The trial researchers had added the 6 mg/kg group, along with a separate placebo group, after people on the highest dose started developing ARIA-E. When results from this later 6 mg/kg group were first presented at the 2015 Alzheimer’s Association International Conference, most researchers were undeterred by the lack of an effect on the MMSE, given that the study was not powered to detect changes in cognition in the first place (see Aug 2015 news). Selkoe added that the CDR-SB is a more sensitive test of cognition than the MMSE anyway.
Lon Schneider of the University of Southern California in Los Angeles could have done without the tentative cognitive findings altogether. For one thing, he pointed out that the placebo group, which was pooled into a single group in the analyses, actually represented three groups that were randomized at different times. “They called it a ‘staggered parallel-group design,’ but really the cohorts were separate parallel-group studies occurring at different times and differing sites,” Schneider wrote to Alzforum. “Without the display of the cognitive data by dosing cohort, one has to wonder whether the apparent CDR-SB and MMSE benefits at 10 mg/kg had to do with variable baselines between cohorts and sites, statistical corrections, or extreme comparisons,” he wrote. Other researchers have expressed similar caution (see Nov 2015 conference news).
Still, Schneider applauded the study’s main findings. “The enthusiasm for aducanumab should rest on its PK, PD, plaque-busting ability, preclinical biochemical characteristics, and clinical safety. One doesn’t need to invoke implausible clinical and cognitive effects to make a compelling case for the potential for aducanumab,” he maintains (see full comment below).
New data in the paper strengthen the connection between amyloid removal and cognitive benefit, Selkoe told Alzforum. They were first reported at the 2015 CTAD conference (see Nov 2015 news). Sevigny and colleagues added a post hoc analysis that suggested performance on both the CDR-SB and the MMSE stabilized only in patients who had a substantial reduction in amyloid at one year. Patients whose Aβ levels did not go down declined cognitively as much as the placebo group. “This result suggests that amyloid removal is key to clinical benefit,” Selkoe said, though he acknowledged the need for Phase 3 results to confirm this.
In an editorial that accompanied the paper, Eric Reiman of Banner Alzheimer’s Institute in Phoenix struck a more cautious tone. He wrote that while the additional cognitive results were encouraging, only confirmatory findings in a Phase 3 trial would suffice to bolster the link between amyloid and cognition, thus supporting the amyloid cascade hypothesis.
Sevigny and colleagues also analyzed amyloid reduction by brain region, finding a dose-dependent reduction in amyloid occurred across all parts of the brain where it accumulates. This brain-wide effect added biological credibility to the findings, Selkoe said.
On safety, the main concern was the dose-dependent ARIA-E, which predominantly affected carriers of the ApoE4 allele. While no one in the placebo group had ARIA-E, it manifested in one (3 percent), two (6 percent), 11 (37 percent), and 13 (41 percent) patients on 1, 3, 6, and 10 mg/kg aducanumab, respectively. A third of those people experienced clinical symptoms associated with ARIA-E, such as headaches. ARIA-E generally emerged in the early days of treatment, and resolved four to 12 weeks later. Nobody who had ARIA-E was hospitalized, and more than half of these participants continued treatment. However, the greatest drop-out rate (38 percent) occurred in the highest dose group, possibly due to the larger number of ARIA-E cases in that group. A quarter of patients in the placebo group discontinued treatment, compared to 23, 19, 17, and 38 percent in the 1, 3, 6, and 10 mg/kg groups, respectively.
The researchers maintained that this uneven distribution of drop-outs across dose groups did not alter the study’s main findings. They conducted a statistical sensitivity analysis that considered the worst-case scenario, namely that the people who dropped out were also the poorest responders, and still remained confident they were seeing true effects, Sandrock told Alzforum at a Nature press briefing. Furthermore, in a post hoc analysis, Sevigny found no significant differences in the extent of amyloid removal or performance on cognitive tests between people with or without ARIA-E.
John Hardy of University College London expressed cautious optimism about the findings. “This was not a Phase 3 study: It was not powered or designed to identify treatment effects, and, even in the context of apparently positive data, we need to consider the possibility of selective subject drop-out and chance as underlying the positive findings. These new data are tantalizing, but they are not yet definitive.”
A long-term extension of the Phase 1b trial as well as a new “titration arm,” in which the dose of aducanumab is gradually ramped up, are still ongoing. The recruitment of 2,700 participants with prodromal or early AD for two Phase 3 studies is underway as well. Given their susceptibility to ARIA-E, ApoE4 carriers in the larger trials will be given lower dose regimens than non-carriers.
“Confirmation that an anti-Aβ treatment slows cognitive decline would be a game-changer for how we understand, treat, and prevent Alzheimer’s disease,” Reiman wrote. “Now is the time to find out.”—Jessica Shugart
References
Therapeutics Citations
News Citations
- Biogen Antibody Buoyed by Phase 1 Data and Hungry Investors
- Barcelona: Antibody to Sweep Up Aβ Protofibrils in Human Brain
- Zuers—Meeting Mixes Translational News and Debate
- Safe at 4 Grams? No ARIA at High Dose of Human Aβ Antibody
- Aducanumab, Solanezumab, Gantenerumab Data Lift Crenezumab, As Well
- Outcomes, Outcomes: Cognition is Crux of New Alzheimer’s Trials
- Gantenerumab, Aducanumab: Bobbing Up and Down While Navigating Currents of Trial Design
Further Reading
Primary Papers
- Sevigny J, Chiao P, Bussière T, Weinreb PH, Williams L, Maier M, Dunstan R, Salloway S, Chen T, Ling Y, O'Gorman J, Qian F, Arastu M, Li M, Chollate S, Brennan MS, Quintero-Monzon O, Scannevin RH, Arnold HM, Engber T, Rhodes K, Ferrero J, Hang Y, Mikulskis A, Grimm J, Hock C, Nitsch RM, Sandrock A. The antibody aducanumab reduces Aβ plaques in Alzheimer's disease. Nature. 2016 Aug 31;537(7618):50-6. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of Southern California Keck School of Medicine
This straightforward, “rite-of-passage,” multiple ascending dose, safety and tolerability study demonstrated that an aducanumab dose range from 3 to 10 mg/kg reduced amyloid fibrils and was safe enough for use in the ongoing Phase 3 pivotal efficacy trials. The extent of ARIA and adverse events show that the 10 mg/kg dose, and possibly the 6 mg/kg dose, may be too high for ApoE ε4 carriers who have detectable levels of amyloid plaques by PET.
The study was most remarkable for demonstrating that aducanumab did exactly what it was engineered to do: clear amyloid fibrils in a predictable, precise, dose-dependent manner (as indicated by the small SEs around the SUVR estimates of change in Figure 2a). This, alone, should cause a high level of enthusiasm.
Unfortunately, and despite the authors’ disclaimer that “[t]he trial was not powered for the exploratory clinical endpoints, thus the clinical cognitive results should be interpreted with caution,” they nevertheless spend a lot of ink analyzing and discussing clinical efficacy claims from a study that was not designed to do so. There are several problems here:
The sponsor treated the three separate and sequential dosing cohorts (placebo, 1, and 3 mg/kg; placebo and 10 mg/kg; placebo and 6 mg/kg cohorts) as though they were a parallel-group trial by comparing the four dosing groups to a placebo group pooled from the three cohorts. That is, they made the study seem as though patients were randomized contemporaneously to placebo or one of the four doses in a parallel-group, dose-ranging trial. In fact, each cohort was separately randomized: The first cohort was randomized earlier than the second, 10 mg/kg cohort; and the second cohort prior to the third, 6 mg/kg cohort. They called it a “staggered parallel-group design,” but really the cohorts were separate parallel-group studies occurring at different times and differing sites. So, for example, the last cohort, the 6 mg/kg dosing group, was compared to mainly placebo patients acquired nearly a year earlier and from different sites.
The above and play of chance might explain why the pooled placebo group tended to score better on the CDR-sb and FCSRT at baseline than the treatment groups. At best, after covariate adjustments for baseline scores and ApoE, and not adjusting the p value for the multiple comparisons, there was a nominally significant effect for the CDR-sb and the MMSE for the 10 mg/kg dose; and this was accompanied by implausibly large mean differences from placebo, about 1.24 and 2.25 for the CDR-sb and MMSE, respectively. Only three of 16 CDR-sb and MMSE contrasts were nominally significant at the unadjusted 0.05 alpha error level, and the NTB and FCSRT (memory) tasks did not show significant effects (Extended Data Table 1). Outcomes from the Cognitive Drug Research (computerized) battery were not reported.
If the individual dosing cohorts had been presented separately as they were in the very similarly designed bapineuzumab MAD study (Salloway et al., 2009), we probably would see substantial variation in the placebo change and clinical effects within each group. Without the display of the cognitive data by dosing cohort, one has to wonder whether the apparent CDR-sb and MMSE benefits at 10 mg/kg had to do with variable baselines between cohorts and sites, statistical corrections, or extreme comparisons.
The enthusiasm for aducanumab should rest on its PK, PD, plaque-busting ability, preclinical biochemical characteristics, and clinical safety. One doesn’t need to invoke implausible clinical and cognitive effects to make a compelling case for the potential for aducanumab.
References:
Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M, Sabbagh M, Honig LS, Doody R, van Dyck CH, Mulnard R, Barakos J, Gregg KM, Liu E, Lieberburg I, Schenk D, Black R, Grundman M, . A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009 Dec 15;73(24):2061-70. PubMed.
St Vincent's Institute
This is very exciting work. The authors included a short discussion on the biochemical characterization of this antibody. The main take-home message is that aducanumab does not recognize monomeric Aβ40 at concentrations approaching 1 micromolar. One of the puzzles about this antibody is how it appears selective for aggregated forms of Aβ and yet recognizes an N-terminal region of Aβ that normally cannot aggregate by itself and is a region that is likely to be highly flexible in aggregated and fibrillar forms of Aβ based on recent structural studies.
Chiesi Farmaceutici S.p.A.
In this paper on the aducanumab Phase 1b study in prodromal and mild AD patients, the data on plaque removal are quite encouraging. As pointed out on AlzForum, the initial positive cognitive data need to be confirmed in the ongoing Phase 3 studies, and I agree with Lon Schneider’s comments on potential biases linked to the staggered design of the study.
In my opinion, there is another source of potential bias in interpreting the cognitive results of the study; it refers to the clinical stage of dropouts. The clinical characteristics of the dropouts are not much detailed in the article, but from Table 1 and Extended Data Figure 2, it seems that 10 of 12 dropouts in the 10 mg/kg group were patients with mild AD at baseline, whereas there were only one or two dropouts among prodromal AD patients in the 10 mg/kg group (see table below). Conversely, in the placebo group the discontinuation rate was similar between prodromal and mild AD patients (21.1 percent versus 28.6 percent). The criteria for definition of prodromal AD were an MMSE of 24-30 (inclusive) and a CDR of 0.5, while for mild AD they were an MMSE of 20-26 and CDR of 0.5-1. Thus, the imbalance in the proportion of prodromal AD and mild AD dropouts in the 10 mg/kg may have contributed to the apparent slow decline observed in this treatment group.
Why there was a much higher dropout rate in mild compared to prodromal AD patients in the 10 mg/kg group? One possible explanation could be a higher incidence of ARIA-E abnormalities in the mild AD group. Compared to prodromal AD, mild AD patients may have a higher Aβ burden in brain vessels and consequently a greater risk of edema or bleeding due to Aβ removal by aducanumab. Interestingly, in the ongoing ENGAGE and EMERGE Phase 3 studies, only patients with MMSE 24-30 and CDR=0.5 at baseline were included.
I hope this may be useful in interpreting the cognitive results of the study.
RIKEN Center for Brain Science
Scientists who have experience isolating Aβ from human brains may find the data shown in Figure 1 puzzling. Our lab has found Aβ deposits in human brain to be as hard as rocks. Because Aβ deposits resist such detergents as SDS, we usually depend on formic acid, an extremely toxic acid, to dissolve them. In general, the concentrations of SDS used for washing human brain tissues and of formic acid for extracting Aβ are 20 percent and 99 percent, respectively. For those unfamiliar with the pathological biochemistry of AD, these concentrations are extremely high. In contrast, you can easily extract plaque Aβ from transgenic mice brain using guanidine hydrochloride. Therefore, one would predict that the effects of immunotherapy on humans and on mice will not resemble each other. Human Aβ deposits are much more difficult to remove by therapeutic antibodies because of their physicochemical properties.
Furthermore, the images in Figure 1 suggest that the nonspecific binding of florbetapir to white matter, which would not be expected to harbor Aβ plaque, is higher before treatment than after. Did the immunotherapy decrease the amount of white matter in the brain?
Scientists including Yasuo Ihara, Colin Masters, Konrad Beyreuther, Steven Younkin, Virginia Lee, and Denis Selkoe would know better than I do, as they have more experience, but I worry that the data in this paper seem a bit too good to be true.
Make a Comment
To make a comment you must login or register.