CRISPR Reveals New Suppressors of α-Synuclein Toxicity
Quick Links
To understand how genes cause complex diseases, researchers usually manipulate them one at a time, by knock-in, knockout, knockdown, or overexpression. That’s fine for a start, but genes don’t act alone. Now, Timothy Lu from the Massachusetts Institute of Technology has developed a CRISPR-based screen that randomly and subtly disrupts the expression of many genes at once. PRISM, short for Perturbing Regulatory Interactions by Synthetic Modulators, resolves suppression of α-synuclein toxicity in yeast, a model for neurodegeneration in Parkinson’s disease, into individual contributions by tens of genes. For a first pass at validating this gene-finding approach, human homologs of the top hits protected neuronal cells from α-synuclein as well. PRISM should be readily translatable to other proteins and more advanced models of neurodegeneration, Lu said. The paper appeared October 5 in Molecular Cell.
- CRISPR-Cas screen modulates transcription of multiple genes at once.
- It reveals tens of yeast genes that suppress α-synuclein aggregation and toxicity.
- Human homologs, alone or in combination, protect neurons.
“The combinatorial power of this technology is awesome,” said Vikram Khurana of Brigham and Women’s Hospital, Boston. “This study gives us a first glimpse of the depths to which we will be able to interrogate biology with this emerging technology.”
Mark Cookson, National Institutes of Health, Bethesda, Maryland, liked that the researchers took a weakness of CRISPR and turned it into a strength. “For gene editing, CRISPR is not absolutely precise,” Cookson told Alzforum. “They’ve used that disadvantage to target multiple genes in one cell. It’s neat thinking,” he said.
For targeted editing of DNA, CRISPR-Cas9 technology relies on small hairpin guide RNAs (gRNAs) that direct the Cas9 nuclease to a complementary sequence in the genome (Sep 2014 news). Variations on the method also allow for targeted modulation of gene expression, where complementary gRNAs usher CRISPR-Cas 9-based transcription factors (crisprTFs) to activate or inhibit specific genes of interest. Lu wondered if he could loosen up that selectivity, so that one gRNA might modulate many genes simultaneously. By screening a library of random sequence guide RNAs, would he be able to find ones that hit multiple genes which together alter a specific phenotype?
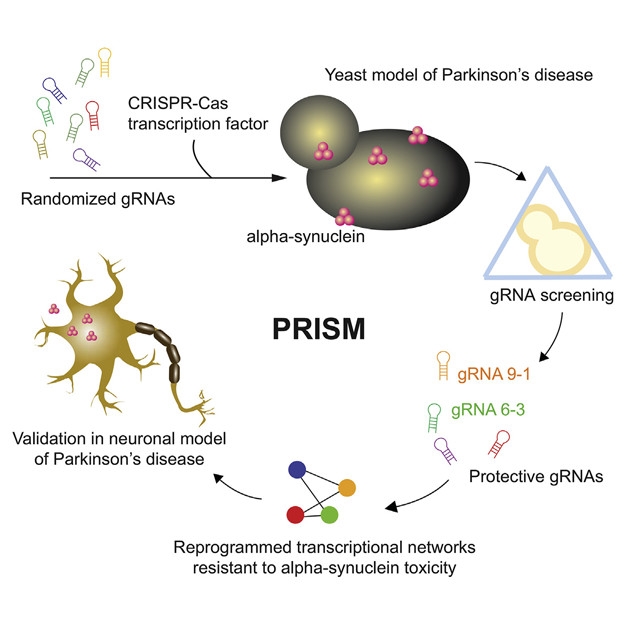
Through a PRISM: Perturbing Regulatory Interactions by Synthetic Modulators enables CRISPR-based transcriptional disruption of multiple genes simultaneously. This unmasks combinatorial genetic effects on α-synuclein toxicity. [Molecular Cell, Chen et al.]
After selecting for yeast carrying at least one of the gRNAs, the authors induced α-synuclein expression. Most of the yeast died. From the survivors, Chen and colleagues followed up on one gRNA, dubbed 9-1, which showed the strongest protective effect. While the yeast genome contained no sequence perfectly complementary to the 9-1 gRNA, there were several dozen sites with just one or two mismatches.
The investigators determined that 9-1 had altered, by at least twofold, the expression of 114 genes. More than 90 percent of them had not been identified in any previous screens for suppressors of α-synuclein toxicity. To systematically validate the genes, the researchers overexpressed them one by one in the α-synuclein yeast strain. Of 95 genes tested, 57 significantly inhibited α-synuclein toxicity, although none alone was as strong as the 9-1 gRNA. In contrast, only five of 34 randomly selected genes had that capacity, indicating that the screen enriched for genes as hoped.
In a second validation assay, the top seven suppressors prevented the formation of cytoplasmic α-synuclein inclusions in yeast, a surrogate for the mislocalization and aggregation seen in human disease. Again, individually the genes were less potent than the 9-1 gRNA.
The new synuclein suppressors fell into familiar functional categories, including protein quality control, ER/Golgi trafficking, lipid metabolism, mitochondrial function, and stress response. Several top hits were related to human genes previously implicated in neurodegenerative disease, including homologs of the Parkinson gene DJ-1/Park7, which is known to attenuate α-synuclein aggregation and toxicity (Zondler et al., 2014).
“The pathways are not a surprise, but the genes are different,” said Khurana. The result has implications for functional genomics. “If we are going to use model organisms to understand human disease, then we don't just need to understand pathways, we need to know the genes so we can interrogate those in patients,” he said.
Using neuroblastoma SH-SY5Y cells engineered to overexpress α-synuclein, the investigators tested human homologs of six of the top yeast hits—DJ-1/PARK7, ALS-2, GGA1, DNAJB1, TXN, and TIMM. Six days of α-synuclein overexpression caused neurite retraction and 50 percent loss of viability. DJ-1/PARK7, ALS-2, GGA1, and DNAJB1 completely or partially prevented these deficits. Neither TXN nor TIMM alone rescued cells, but in combination they completely suppressed synuclein toxicity in the assay. The protection appeared specific for α-synuclein toxicity, because the genes did not protect cells from the dopaminergic toxin MPP+.
At the outset, Lu wondered if gRNAs which modulate many genes simultaneously would give stronger protection than any one gene on its own. The answer appeared to be yes—9-1 was more effective at blocking α-synuclein toxicity and aggregation than any of the single genes or combinations tested, and was also better than the ubiquitin-specific protease, UBP3, a strong suppressor of synuclein toxicity (Cooper et al., 2006). This round of PRISM did not identify this protease gene, nor many suppressor genes previously identified in yeast. Lu pointed out that they screened only a small fraction of the total of 1 trillion possible randomized gRNA sequences, so there could be many more undiscovered hits in their library. “We have now adapted this approach to other models and have shown in other contexts that we can pick out guide RNAs that have unexpectedly strong effects on a phenotype,” Lu said.
However, he admits that the underlying mechanism of protection remains murky. “Our hypothesis at this point is it’s a diffuse or combinatorial modulation of a bunch of targets, but figuring that out is difficult with existing technologies,” he told Alzforum. Going forward, his group will take a systems approach, going beyond the behavior of single genes or pairs of genes and focusing on how the overall state of gene expression in a cell determines its phenotype. That’s similar to the way several groups have looked at gene expression in tissues from AD and FTD patients (May 2013 webinar; Sep 2011 news).
Khurana is interested to see how the screen turns out in yeast models of Aβ, TDP-43, and polyglutamine repeat proteins, saying, “It would be good to see how unique these genes are to this particular proteotoxic stress versus another.”
For next steps, Lu will screen gRNA libraries in more advanced models of disease using neurons derived from induced pluripotent stem cells. Cookson agreed that screening human cells is important and achievable, especially to get a look at genes related to neurotransmitters and synaptic structures, which are not present in yeast.
Lu’s lab is also working to validate the existing hits in additional disease models. “This is my lab’s first foray into neurodegeneration, so we are looking for opportunities to collaborate,” he said.—Pat McCaffrey
References
News Citations
- CRISPR Gene Editing—Poised to Revolutionize Neuroscience?
- Systems Biology Approaches Get Wnt of Progranulin’s Role in FTD
Webinar Citations
Paper Citations
- Zondler L, Miller-Fleming L, Repici M, Gonçalves S, Tenreiro S, Rosado-Ramos R, Betzer C, Straatman KR, Jensen PH, Giorgini F, Outeiro TF. DJ-1 interactions with α-synuclein attenuate aggregation and cellular toxicity in models of Parkinson's disease. Cell Death Dis. 2014 Jul 24;5:e1350. PubMed.
- Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, Liu K, Xu K, Strathearn KE, Liu F, Cao S, Caldwell KA, Caldwell GA, Marsischky G, Kolodner RD, Labaer J, Rochet JC, Bonini NM, Lindquist S. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science. 2006 Jul 21;313(5785):324-8. PubMed.
Further Reading
Papers
- Khurana V, Chung CY, Tardiff DF. From Yeast to Patients: The Audacity and Vision of Susan Lindquist. Cell Syst. 2017 Feb 22;4(2):147-148. PubMed.
Primary Papers
- Chen YC, Farzadfard F, Gharaei N, Chen WC, Cao J, Lu TK. Randomized CRISPR-Cas Transcriptional Perturbation Screening Reveals Protective Genes against Alpha-Synuclein Toxicity. Mol Cell. 2017 Oct 5;68(1):247-257.e5. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.