Is Shape of Aβ an Early Blood Biomarker for Alzheimer’s?
Quick Links
Within the past nine months, researchers developed assays that correlate reduced concentration of soluble Aβ in the blood with amyloid plaques in the brain. Now a group led by Klaus Gerwert, Ruhr-University Bochum, Germany, suggests that changes in the structure of plasma Aβ might herald Alzheimer’s disease. Using infrared spectroscopy to measure the ratio of β-sheet to α-helical forms of Aβ in plasma, the researchers distinguished people with prodromal AD from healthy controls. In a longitudinal study, normal people who tested positive for the β-sheet form were nearly eight times more likely to be diagnosed with Alzheimer’s during the next eight years. Gerwert published the findings in the April 6 EMBO Molecular Medicine.
- Infrared sensor detects β-sheet structures in Aβ peptides from blood.
- The signal can discriminate prodromal AD from controls.
- Positive-testing individuals were nearly eight times more likely to develop AD, an average of eight years later.
"The results with this novel technique are very encouraging,” wrote Colin Masters, University of Melbourne, Australia. Hilal Lashuel, École Polytechnique Fédérale de Lausanne, Switzerland, also welcomed the findings. “It is a very promising development in the right direction, moving beyond measuring total levels [of Aβ] to detecting and quantifying pathogenic species,” he said. “However, it still requires refinement to improve sensitivity.”
For decades, scientists struggled to measure a difference between levels of Aβ peptide in the plasma of patients and controls (April 2016 news; Blennow and Zetterberg, 2018). Success finally came in the form of mass spectrometry-based assays, which increased accuracy and precision over previous measures, and an ELISA (Jul 2017 conference news; Feb 2018 news; Fandos et al., 2017).
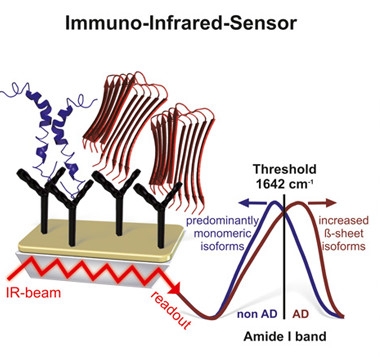
Shape-Shift Sensor.
Antibodies trap α-helical (blue) and β-sheet (red) conformations of Aβ from the blood (left). The β-sheet form, detected by an infrared spectral shift (right), is indicative of AD. [Courtesy of Nabers et al., EMBO Mol Med, 2018.]
Previously, Gerwert and colleagues used infrared spectroscopy to search for the β-sheet type of Aβ found in fibrillar plaques associated with AD. They capitalized on a change in the vibration of amide bond carbonyl groups as the peptide morphs from α-helix to β-sheet. This changes absorption of infrared light, which manifests as a shift within the infrared spectrum (see image at right). This approach enabled Gerwert to discriminate between blood samples from healthy controls and AD patients with a sensitivity of 75 percent and a specificity of 88 percent (Nabers et al., 2016; Nabers et al., 2016).
Now co-first authors Andreas Nabers, Ruhr-University Bochum, and Laura Perna, German Cancer Research Center (DKFZ), Heidelberg, used the same method to test samples from people at earlier disease stages. As in the 2016 study, they used a pan-Aβ antibody, β-Amyloid [13-28], to capture, in theory, all Aβ peptides, including α-helical monomers and protofibril oligomers, and then measured the infrared spectrum of the trapped material. They first examined blood from the prospective Swedish Biomarkers for Identifying Neurodegenerative Disorders Early and Reliably study. BioFINDER samples came from 36 patients with mild cognitive impairment who had abnormal [18F]-flutemetamol PET scans, and 37 cognitively healthy controls with normal amyloid scans.
In the prodromal AD samples, infrared absorption indicated Aβ sheet, as expected (image below). The spectral shift distinguished PET-positive people from PET-negative with a sensitivity and specificity of 69 and 86 percent, respectively.
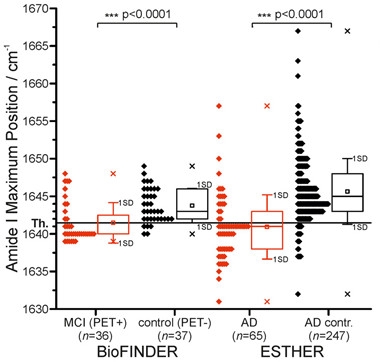
Predicting AD.
A shift in plasma Aβ amide I absorption distinguished PET-positive from PET-negative volunteers (left), and AD patients from controls (right). [Courtesy of Nabers et al., EMBO Mol Med, 2018.]
Next, Nabers and Perna tested earlier stages of disease using baseline samples collected between 2000 and 2002 from ESTHER, a population-based longitudinal cohort of nearly 10,000 healthy older adults from Germany. Follow-up questionnaires, medical records, and biological samples were collected every three years, and incident dementia recorded. During an average of eight years of follow-up, 70 volunteers were diagnosed with AD, of whom 65 had a clean baseline blood sample and an unambiguous diagnosis. Nabers and Perna matched these 65 cases with 247 controls based on age, gender, and education level, and compared their blood Aβ spectra. The assay distinguished AD sample from controls with a sensitivity and specificity of 71 and 91 percent, respectively. Overall, people who were diagnosed with AD were 7.9 times more likely to test positive.
The authors also compared samples from 66 cases of vascular dementia and 36 cases of mixed dementia with 311 and 149 matched controls, respectively. Both sets of samples tested negative. Gerwert cautioned, however, that the difficulty of diagnosing vascular and mixed dementia limits the interpretation of these results.
“This is a completely new aspect of Aβ monitoring,” said Henrik Zetterberg, University of Gothenburg, Sweden. He also noted that the results add to a growing number of studies indicating that Aβ measured in blood is relevant to what is happening in the brain.
The overall accuracy of the test is moderate, however. Gerwert predicts better performance with technical tweaks, including better antibodies to trap Aβ. Zetterberg noted that the motley collection of peptides captured by the antibody used may explain the large overlaps between groups. He said antibodies specific for aggregate-prone Aβ42 may yield cleaner data. Although the signal might be too low to detect in plasma, it could work in CSF, he predicted. Lashuel had a different take. He liked the concept of capturing all forms of Aβ, but noted that the epitope recognized by β-Amyloid [13-28] may be buried in some Aβ configurations. “The low sensitivity of the assay might be due to missing Aβ species,” he said. He suggested reconstituting known Aβ structures in vitro, and then testing if they are recognized by the sensor. Masters wondered if β-Amyloid [13-28] might be capturing not only Aβ, but other proteins bound to the peptides. Gerwert said he cannot exclude this possibility but, based on his earlier work, thinks it unlikely.
The source of the β-sheet-enriched peptides in blood also puzzled some. Katsuhiko Yanagisawa, National Center for Geriatrics and Gerontology in Aichi, Japan, wondered whether the peptides derive from the brain or from peripheral cells.
Zetterberg noted the assay may be capturing a transition from a benign to toxic Aβ conformation that maybe relevant beyond a plasma assay. He thinks such a transition may explain why Aβ42:Aβ40 ratios in human CSF fall into two separate groups, high and low, and rarely in between. “There’s a lack of people in the gray zone,” he said. “This must mean that something happens quickly, perhaps within months, to deplete CSF Aβ42 even though amyloid build-up is gradual.” That something could be a switch to a soluble β-sheet formation, said Zetterberg.
Going forward, all agreed that replication and validation are needed if the test is to be used for trial selection or screening for probable AD prior to more expensive tests, such as amyloid PET.—Marina Chicurel
References
News Citations
- Meta-Analysis of 21 Years of Alzheimer's Fluid Biomarker Research
- Finally, a Blood Test for Alzheimer’s?
- Closing in on a Blood Test for Alzheimer’s?
Antibody Citations
Paper Citations
- Blennow K, Zetterberg H. The Past and the Future of Alzheimer's Disease Fluid Biomarkers. J Alzheimers Dis. 2018;62(3):1125-1140. PubMed.
- Fandos N, Pérez-Grijalba V, Pesini P, Olmos S, Bossa M, Villemagne VL, Doecke J, Fowler C, Masters CL, Sarasa M, AIBL Research Group. Plasma amyloid β 42/40 ratios as biomarkers for amyloid β cerebral deposition in cognitively normal individuals. Alzheimers Dement (Amst). 2017;8:179-187. Epub 2017 Sep 12 PubMed.
- Nabers A, Ollesch J, Schartner J, Kötting C, Genius J, Hafermann H, Klafki H, Gerwert K, Wiltfang J. Amyloid-β-Secondary Structure Distribution in Cerebrospinal Fluid and Blood Measured by an Immuno-Infrared-Sensor: A Biomarker Candidate for Alzheimer's Disease. Anal Chem. 2016 Mar 1;88(5):2755-62. Epub 2016 Feb 16 PubMed.
- Nabers A, Ollesch J, Schartner J, Kötting C, Genius J, Haußmann U, Klafki H, Wiltfang J, Gerwert K. An infrared sensor analysing label-free the secondary structure of the Abeta peptide in presence of complex fluids. J Biophotonics. 2016 Mar;9(3):224-34. Epub 2015 Mar 23 PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Nabers A, Perna L, Lange J, Mons U, Schartner J, Güldenhaupt J, Saum KU, Janelidze S, Holleczek B, Rujescu D, Hansson O, Gerwert K, Brenner H. Amyloid blood biomarker detects Alzheimer's disease. EMBO Mol Med. 2018 May;10(5) PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.