To Block Tau’s Proteopathic Spread, Antibody Must Attack its Mid-Region
Quick Links
Remember the epitope debate around therapeutic Aβ antibodies? N-terminal versus mid-region versus C-terminal—which would be most effective? At the Advances in Alzheimer’s and Parkinson’s Therapies Focus Meeting (AAT-AD/PD), held March 15–18 in Turin, Italy, a similar conversation erupted for tau antibodies. Alzheimer’s researchers are increasingly targeting tau. Neurofibrillary tangle pathology correlates with cognitive decline, but tau also is thought to occur as small, misfolded aggregates that researchers want to catch to interrupt templated seeding and spreading through the brain. Several tau therapeutic antibodies are in development, from preclinical stages to Phase 2, and many groups are trying to figure out which antibody characteristics may work best. In Turin, multiple groups reported that antibodies directed against the middle region of tau stopped seeding of tau extracted from AD brain in cellular assays and mouse models. By contrast, antibodies against the N terminus—which are known to clear neurofibrillary deposits—poorly inhibited seeding and propagation of AD-derived tau in these models. Most tau antibodies currently in the clinic target the N terminus, causing hallway debates about their chance of success.
- In cell culture and mice, antibodies to tau’s N terminus poorly block seeding and propagation.
- Antibodies against tau’s mid-region are more active in these functional assays.
- Several tau antibodies now in trials target the N terminus.
Other researchers cautioned that it remains unclear whether the finding will hold up in AD brain, which contains different forms of tau than do the cellular and mouse models used. “Ongoing clinical trials will start to give answers to this,” Marc Mercken at Janssen told Alzforum. Meanwhile, Luc Buee at the University of Lille, France, urged that more preclinical work be done in multiple experimental models of propagation before advancing antibodies to human trials. “We don’t want to go too fast and make mistakes,” Buee said.
Researchers believe that neurons pass misfolded tau to nearby cells, allowing the pathology to invade anatomically connected brain regions (Mar 2013 conference news; Aug 2013 news; Nov 2016 news). If so, antibodies might be able to intercept these toxic forms in the extracellular space, halting the advance of the disease. Several groups have generated antibodies that target extracellular, aggregated forms of tau, with those from Biogen, AbbVie, and Roche currently the farthest along in development (Apr 2017 conference news; Aug 2017 conference news). Complicating these efforts, however, is the fact that researchers still do not know exactly which species of tau transmit pathology.
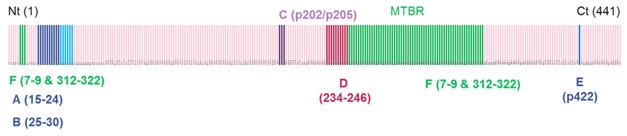
Location, Location. Experimental antibodies “D” and “C,” which bind to the mid-region of tau (red and purple bands), are highly active in seeding assays, while N-terminal antibodies “A” and “B” (binding at blue and navy bands) act weakly. [Courtesy of Jean-Philippe Courade.]
To start to answer this question, researchers at UCB Biopharma in Brussels tested the efficacy of a broad spectrum of tau antibodies. In Turin, Jean-Philippe Courade described the strategy. Led by Martin Citron, the UCB team generated dozens of antibodies by immunizing animals with recombinant tau fibrils bearing different post-translational modifications, as well as paired helical filaments (PHFs) from AD brain. They purified 94 of these antibodies and used surface plasmon resonance to determine what region of tau each one bound. The researchers selected 51 for screening in a functional assay. For the assay, they expressed human P301S tau in HEK 293 cells and added pathological tau isolated from pooled human AD samples to trigger aggregation. Antibodies were added at the same time as the toxic tau. Two days later, the researchers measured the amount of fibrillar tau in the cells by fluorescence resonance energy transfer (FRET).
Only one of the antibodies, UCB0107, strongly suppressed aggregation, Courade reported. UCB0107 targets amino acids 235–246, which lie at the end of tau’s second proline-rich region, right before its microtubule-binding domain. UCB0107 dose-dependently inhibited fibrillization, achieving nearly 100 percent inhibition at an antibody concentration of 300 nM, Courade said. It performed equally well against pathologic tau isolated from progressive supranuclear palsy and frontotemporal dementia tissue samples, he added. UCB is advancing this antibody to the clinic and has started a Phase 1 trial.
Other antibodies only partially suppressed seeding. For example, one that targeted phosphorylated S202/T205 in the mid-region of tau potently lowered seeding, but reached a plateau at around two-thirds inhibition. This suggests that not all seeds are phosphorylated at this site, and points to heterogeneity in the phosphorylation of seeds, Courade said.
Notably, antibodies against the N-terminal region of tau had little effect on seeding, Courade said. The first wave of tau antibodies currently in trials target this region because they were chosen based on affinity, and antibodies tend to bind most strongly here. For a side-by-side comparison, UCB researchers synthesized antibodies with the same complementarity-determining region (CDR) as existing antibodies based on the patent literature and tested them in their seeding assay. They found partial activity for AbbVie’s C2N 8E12, but no activity for Biogen’s BIIB092, which recognizes N-terminal fragments of tau. “The highest-affinity antibodies were not the most efficacious in this assay,” Courade said. He believes the binding epitope is a more important factor for clinical efficacy than affinity.
Researchers at Janssen Pharmaceuticals in Beerse, Belgium, reported similar findings using a slightly different assay. They also generated a panel of antibodies from a variety of antigens, including recombinant aggregated tau and PHFs from AD brains. Unlike UCB, they used these antibodies to immunodeplete pathogenic tau from AD and transgenic mouse brain extracts. Then they added the immunodepleted extract to cells expressing P301S tau, and measured fibrillization.
At AAT-AD/PD, Janssen’s Kristof Van Kolen reported that antibodies against tau’s mid-region best removed pathogenic seeds, whereas antibodies to the N-terminal region of tau only weakly suppressed seeding. The difference was particularly pronounced for AD brain extract, less so for mouse brain extract.
Why don’t N-terminal antibodies stop seeding? “We believe this is due to N-terminal truncation of aggregated tau,” Mercken told Alzforum. The N-terminal tails stick out from the clumped core of tau fibrils and are known to be clipped off by proteases. This means the proteopathic tau seeds may not have the tau N-terminus anymore. Human tau fibrils may be particularly vulnerable to proteolysis because tangles linger for decades in the brain, giving the process more time to occur than in mouse brain, Mercken suggested. Still, he believes some percentage of aggregated tau retains its tails, explaining the partial activity of N-terminal antibodies.
The Janssen team did not report side-by-side comparisons in Turin, but told Alzforum that they saw some efficacy for both C2N 8E12 and BIIB092 in their assays, in contrast to UCB’s results. Janssen also took an antibody active against seeding into Phase 1.
The same pattern occurred in vivo, Mercken told Alzforum. The researchers injected AD brain material enriched for PHFs into the brain of a transgenic tau mouse to spur fibrillization. When they co-injected various antibodies, they again found that those that recognized the mid-region of tau were most effective, while N-terminal antibodies could not fully block seeding.
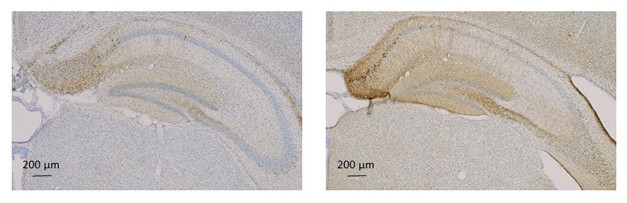
Preventing Seeding. AD brain lysate triggers accumulation of toxic tau (brown, stained with AT8) in the hippocampi of tau transgenic mice (right). This is reduced by intraperitoneal treatment with mid-region tau antibody UCB0107 (left). [Courtesy of Luc Buee and UCB.]
Buee and colleagues reported similar findings from mouse models. The French researchers injected pathological tau from AD brain into the hippocampi of young tau transgenic mice to trigger pathology. This was followed by six weekly intraperitoneal tau antibody injections. In Turin, Morvane Colin in Buee’s group said that a mouse version of UCB0107 prevented tau aggregates from forming.
While the results suggested that UCB0107 inhibits seeding in vivo, it was unclear if the antibody could also stop the spread of misfolded tau once present, Colin noted. To tease this out, the researchers used a different mouse model. In this paradigm, they injected an aggregated tau fragment, K18, which consists of only the microtubule-binding domain. Because UCB0107 does not recognize this fragment, it could not prevent these seeds from triggering local tau aggregation. But injected UCB0107 did prevent tau fibrils from appearing on the opposite side of the brain. “This suggests the antibody stops the spread of pathogenic species,” Colin told Alzforum.
“The message from these studies is that you have to characterize where on tau therapeutic antibodies bind,” Rakez Kayed of the University of Texas Medical Branch, Galveston, told Alzforum. While the first-generation therapeutic tau antibodies were mostly N-terminal, newer ones typically bind the mid-region, he added. One old mid-region antibody is MC1, generated as an experimental tool by Peter Davies of the Litwin Zucker Center for Alzheimer's Research, Long Island, New York (Jicha et al., 1997); it is now being developed as a therapeutic in collaboration with Eli Lilly.
However, speakers were careful to emphasize that it is unclear whether these findings will translate to human brain. The brain lysates used in these assays contain both intracellular and extracellular tau, and so may not resemble the seeds that spread Alzheimer’s pathology, Mercken said. Figuring out which forms of toxic tau are extracellular will be crucial for therapy. For example, Mercken speculated that toxic S422 p-tau may remain intracellular, perhaps explaining the failure of the Roche RG7345 antibody that targeted this form. Buee raised a different concern, suggesting that some of the N-terminal truncation of tau seen in the postmortem human and mouse brain lysates might occur after death. If so, N-terminal antibodies could be more effective in patients than in these assays, he suggested.
Tau mouse models have another limitation, Randall Bateman of Washington University in St. Louis pointed out. They express tau mutations, such as P301S, that predispose people to frontotemporal dementia, not AD. Thus, they may not model the tau pathology present in Alzheimer’s disease, Bateman said. He noted that a robust AD tauopathy model is a needed tool that has yet to be developed. In the meantime, he suggested using models that express wild-type mouse tau.
John Trojanowski and Virginia Lee of the University of Pennsylvania, Philadelphia, have found that, just as was seen in transgenic animals, injection of toxic tau aggregates into wild-type mice causes misfolding and propagation of endogenous tau along the anatomical connectome of the injection site (Nov 2017 news). Trojanowski and Lee reported further details of these studies at AAT-AD/PD.
In addition, factors other than epitope affect the therapeutic efficacy of a given antibody. Mercken said that, in his team’s hands, antibodies against specific tau phosphorylation sites had different activity for human and mouse brain lysate. “That has to be taken into account if you want to progress a phosphorylation-specific antibody to the clinic,” he told Alzforum.
Finally, some antibodies recognize monomeric tau as well as aggregates. In Turin, Trojanowski cautioned that antibodies that bind tau monomers, as does UCB0107, might have to be administered at high doses to prevent physiological tau from soaking up all of the therapeutic. Mercken agrees this is a concern, noting that Janssen has selected an antibody that recognizes phosphorylated PHFs, but not physiological tau, to take into Phase 1. However, Courade suggested that monomeric tau exists at low concentration in the extracellular space, making this less of an issue.—Madolyn Bowman Rogers
References
News Citations
- Tau, α-Synuclein Spread: Crazy Stuff—How Might It Work?
- Tales of Traveling Tau: Is Transfer Between Neurons Normal?
- More Evidence That Distinct Tau Strains May Cause Different Tauopathies
- Treating Tau: Finally, Clinical Candidates Are Stepping into the Ring
- High-Dose Aβ and Tau Immunotherapies Complete Initial Safety Tests
- Human Tau Strains Propagate Faithfully in Wild-Type Mice
Therapeutics Citations
Paper Citations
- Jicha GA, Bowser R, Kazam IG, Davies P. Alz-50 and MC-1, a new monoclonal antibody raised to paired helical filaments, recognize conformational epitopes on recombinant tau. J Neurosci Res. 1997 Apr 15;48(2):128-32. PubMed.
External Citations
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.