Can Antibody-Based PET Scans Pinpoint Aβ Oligomers in the Brain?
Quick Links
Amyloid PET allows clinicians to detect plaque buildup in the brain in living people, but the use of this type of imaging for diagnosis is limited because plaques correlate poorly with disease progression and cognitive status. Soluble, oligomeric forms of Aβ better predict synaptic dysfunction and neurodegeneration, but these tiny structures cannot be seen with current PET ligands. In the February 19 Nature Communications, researchers led by Stina Syvänen and Lars Lannfelt at Uppsala University, Sweden, now report live imaging of these Aβ protofibrils in the brains of transgenic mice using a radiolabeled antibody directed against oligomeric Aβ. The authors used the transferrin receptor to ferry this large protein into the brain. In two different AD mouse models, ligand binding increased with age and correlated with levels of soluble Aβ, suggesting the technique does label this particular form of amyloid. “I think this serves as an important proof of concept that you can do immunoPET in the brain,” first author Dag Sehlin told Alzforum.
Commenters were intrigued by the approach, while noting that the research has a long way to go before it will be practical for people. “This is a great start for the next generation of PET amyloid imaging agents, and it’s encouraging that they see good signal in the brain,” said Joy Zuchero at Denali Therapeutics, South San Francisco. Chet Mathis, who co-discovered the fibrillar Aβ ligand PiB at the University of Pittsburgh, was cautious. “It’s not going to be easy to translate this to humans. There are major hurdles that have to be overcome,” he told Alzforum. These include greatly increasing brain uptake of the tracer while lowering radioactivity to make the technique safe enough for human use, he said.
Voila Protofibrils.
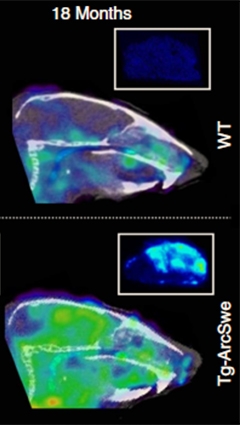
An antibody against soluble Aβ protofibrils (green) lights up the brains of 18-month-old transgenic mice (bottom), but not wild-type (top). [Courtesy of Sehlin et al., Nature Communications.]
Most amyloid PET ligands are small molecules that diffuse readily into the brain. However, because they can bind more than one structure, many poorly discriminate between similar proteins, or different configurations of the same protein. Antibodies recognize specific antigens, but are too large to easily enter the brain. Scientists at the University of California, Los Angeles, pioneered the technique of conjugating large molecules to transferrin receptor (TfR) antibodies. TfR occupies the endothelial cell membrane and normally conducts transferrin from blood to brain, so proteins fused to anti-TfR can hijack this transport system to sneak into the central nervous system (see Wu and Pardridge, 1999). Recently, this idea has been adopted to ferry antibodies into the brain for potential therapeutic purposes (see May 2011 news; Jan 2014 news; Nov 2014 news).
The Swedish researchers wondered if the same method could be employed to shuttle PET antibody tracers into the brain. The authors collaborated with BioArctic Neuroscience, Stockholm, a biotech company founded by Lannfelt, to develop the monoclonal antibody BAN2401, currently in a Phase 2 trial as an anti-amyloid therapy (see Apr 2011 conference news; Nov 2012 conference news). The antibody selectively recognizes soluble, protofibrillar forms of Aβ.
The Swedish researchers fused a fragment of the antibody to the anti-TfR antibody 8D3, then labeled the construct with iodine-124. This fusion protein reached 15-fold higher brain concentrations in mice than did BAN2401, demonstrating the transport strategy worked to increase brain uptake.
To determine how well the tracer detects Aβ, the authors administered the ligand to TgArcSwe and wild-type mice at four, 12, and 18 months of age. Then they waited three days before sacrificing the mice and measuring radioactivity in the brain and blood with a gamma counter. The signal was higher in transgenic brains at 12 and 18 months, and correlated closely with the levels of soluble Aβ, but not total Aβ, as measured by ELISAs. Nuclear emulsion autoradiographs revealed that the tracer tended to cluster around the periphery of plaques, where soluble aggregates have been reported to gather, again suggesting it selectively detected soluble forms of the peptide.
The authors then scanned 13 live TgArcSwe mice and 13 wild-type animals of varying ages in a mouse PET scanner. They waited 72 hours after injection to allow the tracer to clear from blood, providing a better brain-to-blood contrast. As with ex vivo experiments, the ligand lit up the brains of 12-month-old and older transgenic mice (see image above). The signal in various brain regions, such as the hippocampus, cortex, and thalamus, matched what would be expected for disease progression in these animals, the authors noted.
For comparison, they also scanned four TgSwe mice. This gave a signal only at 18 months, which is in keeping with the slower development of pathology in this model (for direct comparison, click the visualizations tab on the Alzforum database page). Notably, the PET signal did not overlap between wild-type and transgenic mice, cleanly delineating these groups. In future studies, the authors plan to test whether the PET signal tracks with cognition and with any improvement after therapeutic use of BAN2401. “That’s one of our main questions,” Sehlin said.
Commenters wondered, however, how well the technique would work in people. “These microPET studies are encouraging, but are hard to extrapolate to humans,” William Klunk at the University of Pittsburgh wrote to Alzforum. He noted that the amount of tracer that entered the brain in these studies was only one-tenth of what would be needed to achieve good images in human brain.
Mathis agreed, adding that in people, it would be impractical to wait several days for the signal to accrue as the author did for these mice. This is because human applications need to use short-lived radionuclides to keep radiation exposure low. Iodine 124 has a four-day half-life compared to two hours for fluorine 18, and imposes a high radiation burden. Scaling up the doses used in mice to the equivalent for the human body would subject people to dangerous levels of radiation, Mathis said. Switching to short-lived radionuclides would require brain penetration to be 10-20 times higher than it was in this study to achieve a visible signal, he added. Zuchero suggested that a lower-affinity anti-TfR antibody might help the construct clear out of endothelium and diffuse into the brain better (see Jan 2014 news).
The authors concur that much remains to be done to make this technique viable in the clinic. Sehlin told Alzforum they are working on improving the pharmacodynamics to develop a tracer that clears from blood more rapidly. This would improve the brain-to-blood ratio and sharpen the signal, as well as allow for faster imaging. In addition, they plan to use a different radionuclide with a shorter half-life. The researchers also need to develop an antibody to human transferrin receptor, as 8D3 recognizes only the mouse receptor, or use a different transport system. All this puts antibody PET for protofibrils still a few years away from clinical studies, Sehlin predicted.
However, Sehlin believes the general approach has broad potential. His group is developing a similar ligand for α-synuclein, but has not yet begun to study it in mice. No α-synuclein tracers have been tested in people yet, though several groups are trying to develop small-molecule ligands. “We hope this concept of using receptor-mediated transcytosis to conduct antibody PET in the brain will be widely used,” Sehlin said.—Madolyn Bowman Rogers
References
News Citations
- Smuggling Antibodies to BACE Across the Blood-Brain Barrier
- Brain Shuttle Ferries Antibodies Across the Blood-Brain Barrier
- Antibody Ferry Looks Safe in Monkeys, Charts Course for Human Studies
- Barcelona: Antibody to Sweep Up Aβ Protofibrils in Human Brain
- CTAD: Adaptive Antibody Trial to Try Bayesian Statistics
- Less Is More: High-Affinity Antibodies Block Blood-Brain Barrier Conduit
Therapeutics Citations
Research Models Citations
Paper Citations
- Wu D, Pardridge WM. Neuroprotection with noninvasive neurotrophin delivery to the brain. Proc Natl Acad Sci U S A. 1999 Jan 5;96(1):254-9. PubMed.
Other Citations
External Citations
Further Reading
Primary Papers
- Sehlin D, Fang XT, Cato L, Antoni G, Lannfelt L, Syvänen S. Antibody-based PET imaging of amyloid beta in mouse models of Alzheimer's disease. Nat Commun. 2016 Feb 19;7:10759. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
New York University School of Medicine
This interesting article relates nicely to our work with tau antibody and its single chain variable fragment (Krishnaswamy et al., 2014).
References:
Krishnaswamy S, Lin Y, Rajamohamedsait WJ, Rajamohamedsait HB, Krishnamurthy P, Sigurdsson EM. Antibody-derived in vivo imaging of tau pathology. J Neurosci. 2014 Dec 10;34(50):16835-50. PubMed.
Make a Comment
To make a comment you must login or register.