Don’t Name It: Glial States Confound Easy Labels
Quick Links
DAM, HAM, WAM, LDAM, MGnD, ARM, A1/A2—got them all straight? The list of names for microglial and astrocyte profiles is growing, because these glial cells change in diverse ways in response to brain pathology. As recent studies have begun to explore this variety of reactive states in depth, an alphabet soup of labels is entering the literature, usually with the first lab discovering a given state also naming it. The trouble is that the second, third, and fourth labs trying to use a new name may find it as confusing as clarifying.
- DAM, HAM, ARM? Scientists are trying to standardize nomenclature for reactive astrocytes and microglia.
- They say: Avoid naming, just describe.
- In so doing, emphasize functional changes over transcriptional profiles.
For example, it is unclear if microglia labeled “DAM” in different mouse models are really the same, or how similar the DAM, MGnD, or ARM microglia identified by different labs are to each other. To try to bring order to this complex landscape of partial overlap, leading scientists in the glial field have come together over the past two years to establish some ground rules for talking about these cells. A group of 81 researchers published a consensus statement on reactive astrocyte nomenclature in the March 2021 Nature Neuroscience, while 91 researchers are finalizing a similar paper on microglial nomenclature, which is in press at Cell and available on Sneak Peek.
Their primary conclusion? For now, authors should avoid slapping names on a glial state they have discovered. Instead, it is better to merely describe the cells in detail, combining transcriptomic, proteomic, morphological, and functional data while also specifying the context where the cells were found—the model, disease, stage, brain region, age, sex, etc. This humble descriptive approach will enable researchers to better compare glial states between studies, the authors said. After a period of accruing data in this way, names will eventually emerge from more solid knowledge base. “At the moment, we know too little to provide a fixed nomenclature or categorization of reactive astrocytes,” noted Carole Escartin at CNRS, Université Paris-Saclay, France, the first author on the astrocyte consensus paper.
Bart De Strooper at the UK Dementia Research Institute, London, a co-author on both papers, thinks these efforts are on the right track. “I like the advice in the two articles to provide a complete description of the cell state under investigation, and to be a bit careful with conclusions based on one approach,” he wrote to Alzforum (full comment below).
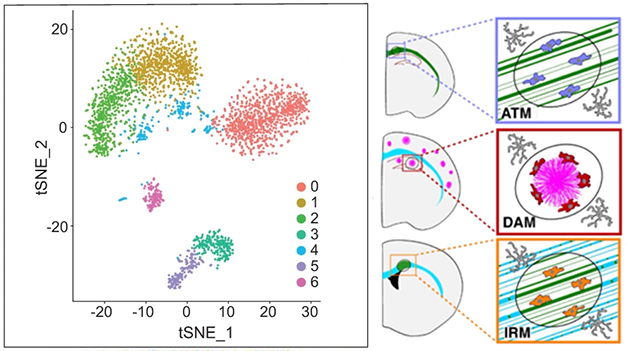
Dramatic Diversity. An RNA-Seq study of astrocytes in a mouse model of multiple sclerosis found seven distinct transcriptional profiles (left). Unique reactive microglial profiles appear in different contexts, such as axon-tract-associated microglia (top right), disease-associated microglia around amyloid plaques (middle right), and injury-responsive microglia in white-matter lesions (bottom right). [Courtesy of Escartin et al., 2021, and Paolicelli et al., 2022.]
Astrocytes: Standardize Terms, Avoid Binary Thinking
In particular, both papers cautioned against the lure of easy dichotomies, of categorizing glial cells as resting versus activated, or protective versus toxic. The more scientists learn, the more apparent it becomes that these labels are oversimplifications of complex and dynamic states, which can be beneficial or detrimental in different contexts. For example, in the astrocyte field, a seminal paper describing two such reactive states, later dubbed A1 and A2, was widely misunderstood as dividing astrocytes into bins of “good” and “bad” (Zamanian et al., 2012; Jan 2017 news). Those names, reminiscent of the discarded microglial categorization into M1/M2, have since been dropped.
Escartin ran into this confusion over terminology while writing a review on reactive astrocytes. This led her to survey several dozen scientists in the field to determine points of consensus and of controversy (Escartin et al., 2019). Following that effort, she brought together the current, larger group to issue recommendations on nomenclature.
Because the group formed at the height of pandemic lockdowns in 2020, they never met in person. Instead, they conferred by email and held online discussions on specific topics. In addition to Escartin, the primary authors were Elena Galea at the Universitat Autònoma de Barcelona, Spain; Michael Sofroniew at the University of California, Los Angeles; and Alexei Verkhratsky at the University of Manchester, U.K.
Specifically, the group suggests investigators retain the umbrella term “reactive astrocytes” to refer to cells that change in response to disease or injury, while reserving “activated astrocytes” to describe physiological responses. They also suggested referring to astrocytes that have a particular molecular and functional profile as a “state,” rather than a “subtype,” to acknowledge the often transient nature of these cellular conditions. “The changes that astrocytes can undergo are extraordinarily subtle. Rather than shifting into a fundamentally different mode, they may just make small changes that are needed at that moment,” Sofroniew told Alzforum.
The authors cautioned against relying on any single marker to define a state. For example, a widely used marker of reactive astrocytes, GFAP, is present in many conditions but does not correlate with the degree of reactivity. Cell shape is also an uncertain indicator. Instead, the authors recommended an emphasis on cell function as the defining feature of a state.
“What ultimately matters is the function. That should be the hallmark we use,” noted co-author Shane Liddelow at New York University. Escartin stressed the importance of confirming functional changes, rather than assuming function based on key molecular markers. “If you want to say astrocytes are neuroprotective or neurotoxic, you need to assess the viability of neurons,” she told Alzforum.
Microglia: Never Resting, Hard to Pin Down
In the microglia field, transcriptomic studies have revealed a dizzying variety of expression profiles in healthy and diseased brains (Feb 2019 news; Nov 2019 news; Dec 2020 news). Such studies underlined that there is no such thing as a “resting” microglia. The cells are always active, probing their environment and responding to changing conditions. Nonetheless, even some 2022 papers still refer to resting and activated microglia, or even M1 and M2.
“If we want to understand microglia, we need to move away from these simplistic categorizations,” Marie-Eve Tremblay at the University of Victoria, Canada, told Alzforum. “The field is growing so fast. We’re afraid new researchers will be misled if we continue like this.”
Tremblay spearheaded the effort to bring this field together, organizing an online workshop with all the authors in June 2021. In addition to Tremblay, the primary authors were Rosa Paolicelli at the University of Lausanne, Switzerland; Amanda Sierra at Achucarro Basque Center for Neuroscience, Leioa, Spain; and Beth Stevens at Boston Children’s Hospital.
As in the astrocyte field, the authors advise avoiding dichotomous labels. In particular, they said “homeostatic” versus “activated” is no longer a meaningful distinction. However, the term “homeostatic” is still useful in relation to a specific context, for example, to compare microglia in a given region of healthy versus diseased brain. Likewise, “disease-associated microglia” (DAM) should be used only for the context in which it was first described, i.e., in response to the presence of amyloid pathology, not as a universal disease signature. Echoing the astrocyte researchers, the microglia experts also emphasized the importance of defining cell states via multiple modalities, with function being the most important.
Shall Glia Remain Nameless Forever?
The authors of both papers acknowledge that passing up names in favor of elaborate descriptions could be challenging. Names do help with quick reference and to discuss findings between labs. Even so, the consensus groups believe the effort will eventually result in a better understanding of glia that could lead to a more rigorous classification system.
“The goal is to generate data on microglial complexity, and eventually we will be able to connect the dots on how microglia in one condition resemble those found in other conditions, and how they differ,” Tremblay told Alzforum. Liddelow agreed. “We need not pigeonhole ourselves now,” he said.
Microglial researchers plan to revisit the issue of nomenclature every five to 10 years, as more information becomes available. “Eventually there could be a white paper where we explain how to name microglia,” Tremblay predicted.
Nonetheless, Escartin cautioned that glia may never fall into neat categories like those of neurons, which are terminally differentiated cells. “Glial cells are too plastic,” she noted.
It appears the guidelines have been well-received. Tremblay said senior investigators at her institution are trying to implement this new descriptive approach in their public talks.
Escartin believes the astrocyte paper has already made a difference. “It is widely read and cited, and I think people are more careful in the terms they use,” she told Alzforum. For example, two recent papers focused on describing the states astrocytes assumed in response to inflammatory stimuli, without naming them (Jun 2022 news).—Madolyn Bowman Rogers
References
News Citations
- Microglia Give Astrocytes License to Kill
- Single-Cell Profiling Maps Human Microglial Diversity, Flexibility
- The Human Brain Hosts a Menagerie of Microglia
- Most Detailed Look Yet at Activation States of Human Microglia
- Astrocyte Reactivity: Opposing States Emerge
Paper Citations
- Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrogliosis. J Neurosci. 2012 May 2;32(18):6391-410. PubMed.
- Escartin C, Guillemaud O, Carrillo-de Sauvage MA. Questions and (some) answers on reactive astrocytes. Glia. 2019 Dec;67(12):2221-2247. Epub 2019 Aug 19 PubMed.
Further Reading
News
- Reactive Astrocytes Boot Basic, Dysfunctional Lysosomes
- WAM! New Microglial Subtype Mops Up Moribund Myelin
- Single-nucleus RNA Sequencing Misses Activation of Human Microglia
- DAMned to Death? Microglia May Proliferate to Senescence
- TREM2 Risk Variant Eggs on Clique of Microglia in AD Brain
- Spatial Transcriptomics Uncovers Coordinated Cell Responses to Amyloid
- Newly Identified Microglia Contain Lipid Droplets, Harm Brain
- Down to Sex? Boy and Girl Microglia Respond Differently
- A Delicate Frontier: Human Microglia Focus of Attention at Keystone
- Girl Power? In Mice, Female Microglia Protect Against Ischemic Injury
- ApoE and Trem2 Flip a Microglial Switch in Neurodegenerative Disease
- Hot DAM: Specific Microglia Engulf Plaques
- Aged Astrocytes Prime Brain for Neuroinflammation
- What Makes a Microglia? Tales from the Transcriptome
- Microglia Reveal Formidable Complexity, Deep Culpability in AD
- Microglia in Tauopathy: Not Just Homeostatic Versus DAM
- Newborn Healers—Novel Astrocytes Repair Brain Injury
Primary Papers
- Escartin C, Galea E, Lakatos A, O'Callaghan JP, Petzold GC, Serrano-Pozo A, Steinhäuser C, Volterra A, Carmignoto G, Agarwal A, Allen NJ, Araque A, Barbeito L, Barzilai A, Bergles DE, Bonvento G, Butt AM, Chen WT, Cohen-Salmon M, Cunningham C, Deneen B, De Strooper B, Díaz-Castro B, Farina C, Freeman M, Gallo V, Goldman JE, Goldman SA, Götz M, Gutiérrez A, Haydon PG, Heiland DH, Hol EM, Holt MG, Iino M, Kastanenka KV, Kettenmann H, Khakh BS, Koizumi S, Lee CJ, Liddelow SA, MacVicar BA, Magistretti P, Messing A, Mishra A, Molofsky AV, Murai KK, Norris CM, Okada S, Oliet SH, Oliveira JF, Panatier A, Parpura V, Pekna M, Pekny M, Pellerin L, Perea G, Pérez-Nievas BG, Pfrieger FW, Poskanzer KE, Quintana FJ, Ransohoff RM, Riquelme-Perez M, Robel S, Rose CR, Rothstein JD, Rouach N, Rowitch DH, Semyanov A, Sirko S, Sontheimer H, Swanson RA, Vitorica J, Wanner IB, Wood LB, Wu J, Zheng B, Zimmer ER, Zorec R, Sofroniew MV, Verkhratsky A. Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci. 2021 Mar;24(3):312-325. Epub 2021 Feb 15 PubMed.
- Paolicelli R, Sierra A, Stevens B, Tremblay ME, Aguzzi A, Ajami B, Amit I, Audinat E, Bechmann I, Bennett M, Bennett F, Bessis A, Biber K, Bilbo S, Blurton-Jones M, Boddeke E, Brites D, Brône B, Brown GC, Butovsky O, Carson MJ, Castellano B, Colonna M, Cowley SA, Cunningham C, Davalos D, De Jager PL, De Strooper B, Dénes Á, Eggen BJL, Eyo U, Galea E, Garel S, Ginhoux F, Glass CK, Gokce O, Gomez-Nicola D, González B, Gordon S, Graeber MB, Greenhalgh AD, and Gressens P, Greter M, Gutmann DH, Haass C, Heneka MT, Heppner F, Hong S, Jung S, Kettenmann H, Kipnis J, Koyama R, Lemke G, Lynch M, Majewska A, Malcangio M, Malm T, Mancuso R, Matteoli M, McColl B, Miron VE, Molofsky AV, Monje M, Mracsko E, Nadjar A, Neher JJ, Neniskyte U, Neumann H, Noda M, Peng B, Peri F, Perry VH, Popovich PG, Priller J, Ragozzino D, Ransohoff RM, and Salter MW, Schaefer A, Schafer DP, Schwartz M, Simons M, Streit WJ, Tay TL, Tsai LH, Verkhratsky A, von Bernhardi R, Wake H, Wittamer V, Wolf SA, Wu LJ, Wyss-Coray T. Defining Microglial States and Nomenclature: A Roadmap to 2030. Cell Press Sneak Peek, March 23, 2022 Cell Press Sneak Peek
Follow-On Reading
Papers
- Paolicelli RC, Sierra A, Stevens B, Tremblay ME, Aguzzi A, Ajami B, Amit I, Audinat E, Bechmann I, Bennett M, Bennett F, Bessis A, Biber K, Bilbo S, Blurton-Jones M, Boddeke E, Brites D, Brône B, Brown GC, Butovsky O, Carson MJ, Castellano B, Colonna M, Cowley SA, Cunningham C, Davalos D, De Jager PL, de Strooper B, Denes A, Eggen BJ, Eyo U, Galea E, Garel S, Ginhoux F, Glass CK, Gokce O, Gomez-Nicola D, González B, Gordon S, Graeber MB, Greenhalgh AD, Gressens P, Greter M, Gutmann DH, Haass C, Heneka MT, Heppner FL, Hong S, Hume DA, Jung S, Kettenmann H, Kipnis J, Koyama R, Lemke G, Lynch M, Majewska A, Malcangio M, Malm T, Mancuso R, Masuda T, Matteoli M, McColl BW, Miron VE, Molofsky AV, Monje M, Mracsko E, Nadjar A, Neher JJ, Neniskyte U, Neumann H, Noda M, Peng B, Peri F, Perry VH, Popovich PG, Pridans C, Priller J, Prinz M, Ragozzino D, Ransohoff RM, Salter MW, Schaefer A, Schafer DP, Schwartz M, Simons M, Smith CJ, Streit WJ, Tay TL, Tsai LH, Verkhratsky A, von Bernhardi R, Wake H, Wittamer V, Wolf SA, Wu LJ, Wyss-Coray T. Microglia states and nomenclature: A field at its crossroads. Neuron. 2022 Nov 2;110(21):3458-3483. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
UK Dementia Research Institute@UCL and VIB@KuLeuven
These two papers bring the two fields of microglia and of astroglia together, as they reflect a broad discussion among different scientists trying to come to an agreement about the nomenclature in their fields. Clearly, the two papers come to a similar conclusion, i.e., that it is too early to decide yet. Both papers are wake-up calls to leave behind simplistic concepts, such as “activated” or “homeostatic”—especially when using only one experimental approach, for instance transcriptomics, to define cell types.
I believe researchers will adapt how they discuss microglia based on these papers. One of the things we need is more complete integration of different information. A lot of progress is based on simple transcriptomics, but that says little about function or morphology. All the information needs to be integrated when trying to understand the function of a particular cell state.
Following these papers, there will be efforts to classify microglia, and astroglia, and to get insight in the relationship between the different cell types. However, I am not sure that we will really see different microglia. My favorite hypothesis currently is that microglia adapt particular “cell states,” and that those reflect their versatility and their capacity to adapt to the needs of the organism. These are not necessarily different “cell types.” To sort this out, we need to know whether microglia can move from one cell state to another, or whether there are predefined groups of cell types that can expand in function in response to external challenges.
For the time being, I like the advice to provide complete description of the cell state that is investigated, and to be a bit careful with conclusions based on one approach.
Universitat Autònoma de Barcelona
I want to elaborate on the suggestion in both articles, on which I am an author, to avoid simplistic binary classifications, such as homeostatic versus activated or neuroprotective versus neurotoxic, when describing the reactions of microglia and astrocytes.
First, it is not only that these cells may adopt numerous states in health and disease, but also that they are highly specialized to act as building blocks in neural circuits, performing many functions that cannot be characterized as "neuroprotection" or "neurotoxicity," as though they were tissue macrophages fighting against infections.
Second, each and every microglial and astrocytic state is arguably the result of a combination of adaptive and maladaptive changes aimed to maintain or restore the roles of these cells and to protect the cells—it is seldom recognized that glia are damaged during diseases, too. If adaptive changes predominate, the states represent resilient phenotypes; since diseases are rare, resilience is probable the prevalent response in a normal life. If, by contrast, maladaptive changes predominate, we should talk about gliopathies and develop therapies aimed to restore original glia functions.
Note that I have managed to talk about glia in CNS diseases without resorting to the term "neuroinflammation." In the astrocyte article, "inflammation" was only used to describe infiltration of immune cells into the brain, whereas in the microglia article, it is explicitly recommended to avoid using "neuroinflammation" as a synonym of microglia responses.
Make a Comment
To make a comment you must login or register.