Of Mice and Magnets—Can We Learn about Alzheimer's from Mini MRIs?
Quick Links
Neural circuits go on the fritz in Alzheimer’s disease, but how that relates to underlying disease mechanisms is unclear. Researchers have identified dysfunctional circuits in the AD brain using magnetic resonance imaging, but they cannot manipulate Aβ and other pathologies in the human brain as they can in model systems. A small group of researchers is now turning giant magnets onto much tinier, more controllable subjects—mice. Though human and mouse brains are wired differently, some researchers believe that mouse functional MRI, when perfected, will help explain how amyloid pathology and neural circuitry problems are intertwined, and could serve as a useful tool to screen potential therapies.
One example was published October 8 in The Journal of Neuroscience. Researchers used functional and structural MRI to reveal that neural circuits falter long before amyloid plaques emerge in a mouse model of AD. Led by Markus Rudin at the University of Zurich, the study is one of few recent attempts to track early changes in functional circuitry in mice brought about by Aβ.
“This is an important new finding that expands upon previous work in both humans and mice, establishing resting-state functional connectivity as a proxy for and predecessor to increased brain amyloid levels,” commented Yvette Sheline of the University of Pennsylvania in Philadelphia, who was not involved in the study. “This offers us the potential to use resting-state functional connectivity to identify the very earliest stages of preclinical Alzheimer's disease, a decade or more before onset of clinical symptoms.”
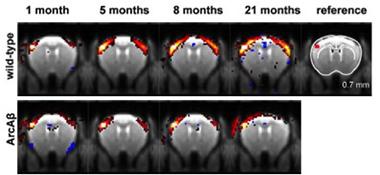
Missed Connections.
Correlations between activity in a reference region in the sensory cortex (top right, red) and other regions of the brain strengthened in wild-type mice early in life (top), but not in ArcAβ mice (bottom). Red and yellow reflects stronger correlations. [Image courtesy of Grandjean et al., The Journal of Neuroscience 2014.]
Functional MRI detects subtle changes in the concentration of oxygenated blood as a proxy for neural activity. Researchers use fMRI to probe the connections between different regions of the human brain. The noninvasive technique has revealed the existence of several neural networks, including the famed default mode network (DMN), which switches on during idleness and has a propensity to accumulate Aβ (see Raichle et al., 2001). Sheline and other researchers used the technique to detect neural disconnections in cognitively normal people who have amyloid plaques, in people who have low CSF Aβ or high p-tau, and in ApoE4 carriers who had no plaque burden (see Hedden et al., 2009; Aug 2013 news story; Dec 2010 news story).
With all this success in humans, why turn to mice? “Discoveries using fMRI in humans have excited the entire AD research community, but they stop short at revealing fundamental mechanisms,” said first author Joanes Grandjean. “It is really difficult to understand the mechanisms underlying these functional changes in humans, because there is such a large diversity of comorbidities among patients, and it is unclear when or if they will develop disease,” he said. Mice give researchers control over their pathology and the opportunity to use a plethora of different models, to test therapies, and to analyze brain tissue for the molecular culprits behind wonky circuits.
Despite these advantages, optimizing functional MRI for small animals has not been easy. Resting-state functional MRI relies on subtle changes in oxygen concentration. Factor in the small size of a mouse brain, and this calls for particularly high spatial resolution and more sensitive equipment, including a strong magnet and precise detectors, Grandjean said. They used a 9.4T magnet for their study, and have posted detailed raw data online so others will have data for comparison. Further complicating matters, animals must be anesthetized during the procedure to prevent movement, and anesthesia inhibits neural activity—a particularly worrisome factor for those seeking to measure it. While rats can reportedly learn to relax while being restrained in an MRI machine (see Liang et al., 2011), awake mice are less cooperative. Researchers are still coming up with ways to improve the accuracy and utility of functional MRI in these teeny mammals.
Initial fMRI studies on mice looked at changes in functional connectivity in the somatosensory cortex in response to a foot shock (see Ahrens and Dubowitz, 2001; Nair and Duong, 2004; and Adamczak et al., 2010). Only more recently have researchers ventured into the realm of measuring resting-state functional connectivity in multiple regions of the mouse brain (see Jonckers et al., 2011). A study led by David Holtzman at Washington University, St. Louis, employed an optical technique, rather than MRI, to measure an uptick in oxygenated hemoglobin as a proxy for neural activity prior to plaque deposition in an APP/PS1 mouse model (see Mar 2012 news story). Later, researchers led by Annemie van der Linden at the University of Antwerp, Belgium, used fMRI to document decreased functional connectivity in older, plaque-ridden APP/PS1 mice (see Shah et al., 2014).
Grandjean and colleagues sought to extend these previous findings—both temporally and spatially—by performing an extensive time course of fMRI measurements in the ArcAβ mouse model, which expresses human APP harboring both the Arctic and Swedish mutations. The researchers used histological staining to confirm the growth of amyloid plaques in a subset of mice throughout the time course of the fMRI study. They observed sparse amyloid deposits in some mice by five months, with full-blown plaques apparent at 11 months. Despite this late plaque development, the researchers found that connectivity problems emerged much earlier. Between one and five months of age, functional connectivity between the left and right parietal and motor cortices ramped up in the wild-type mice, whereas the transgenics forged these connections at a snail’s pace. For example, in wild-type mice the strength of bilateral connections across the somatosensory cortex more than doubled between one and five months of age, whereas in ArcAβ mice the connections stagnated at one month.
The researchers saw no strong functional connectivity in the hippocampus or other subcortical regions in this model. This was likely due to the use of isofluorane as an anesthetic, Grandjean said. Isofluorane inhibits GABAergic neurons, which are more prominent in the hippocampus than the cortex. In a parallel study, the researchers corrected this by combining smaller amounts of two different anesthetics, allowing functional connectivity measurements in both the cortical and subcortical regions, including the hippocampus (see Grandjean et al., 2014).
In addition to functional measurements, the researchers used diffusion tensor imaging MRI to monitor the development of white-matter tracts between different brain regions. Similar to the fMRI results, the white-matter tracts in wild-type mice grew thicker between one and five months of age, whereas those in ArcAβ largely stayed the same.
The results suggested that a breakdown in both functional and structural connectivity occurred far in advance of plaque deposition. This hints that earlier forms of pathology, perhaps soluble Aβ oligomers, play a much stronger role in bungling neural circuitry than do amyloid plaques that develop later, Grandjean said.
How might soluble Aβ oligomers weaken connections? Grandjean and Rudin hypothesized that the peptides could reduce synaptic activity and/or prevent the proper guidance of axons as the brain develops. They plan to compare how connectivity matures in mouse models with varying pathologies. For example, the ArcAβ model harbors both intra- and extracellular amyloid plaques and develops vascular pathology, while the APP/PS1 models only sprout extracellular plaques and do not develop significant vascular pathology, Grandjean said. Another mouse model expressing APP harboring the Osaka mutation only develops intracellular Aβ oligomers (see Umeda et al., 2012). Grandjean and Rudin want to compare as many models as possible to tease out the underlying mechanisms that may cause a breakdown in connectivity.
A less-satisfying explanation for the dip in connectivity seen in APP mice—and one that would make them less ideal as models for human disease—could be that early overexpression of APP triggers developmental defects, suggested Rylan Allemang-Grand of the University of Toronto. This is a common concern in the field. For example, overexpression of APP might gum up presenilin 1, preventing the enzyme from processing Notch1, which is important for development (see Berezovska et al., 2001). Allemang-Grand recently used manganese-enhanced MRI, or MEMRI, to monitor neuroanatomical development in an aggressive mouse model of AD, TgCRND8. The manganese works as a contrast agent to allow the visualization of subtle anatomical features. He found early anatomical differences between wild-type and TgCRND8, far in advance of plaques (see Allemang-Grand et al., 2014). Whether these are due to APP overexpression, or to some other pathology, such as soluble Aβ oligomers, is unclear, he said. Using conditional mouse models that switch on APP only after the brain has developed (such as TetO-APPSweInd) would be one way to resolve the issue, Allemang-Grand said.
Another issue researchers will need to contend with when measuring connectivity in mice is how well mouse functional networks compare to those in people. While connections between regions of the DMN are the first to falter in AD, in Grandjean’s study the ArcAβ somatosensory cortex developed the earliest and most obvious connectivity failures. Like the human DMN, the mouse somatosensory cortex is one of the most metabolically active regions in the brain and the most functionally connected. Given that neural activity may precipitate release of Aβ, disruptions in the DMN and the somatosensory network could reflect a common pathology in mice and humans, the authors suggested. “More active regions release more soluble Aβ, which breaks down the functional networks and leads to amyloid plaques later in life,” Grandjean said.
Disha Shah of the University of Antwerp, Belgium, who led another recent study comparing functional connectivity in APP/PS1 mice, said Grandjean and colleagues took a step closer to identifying early functional disconnects. She cautioned, however, that more details need to be ironed out. “Conclusions drawn from any resting-state fMRI studies must take into account the characteristics of the mouse model and the anesthesia regime that is used,” she said. “Therefore, studies in different mouse models of amyloidosis with different patterns of disease progression and the use of different anesthesia regimes might provide even more insight into which brain regions are specifically vulnerable and how this relates to amyloid pathology.”—Jessica Shugart
References
News Citations
- Brain Connectivity Reveals Preclinical Alzheimer’s Disease
- A Foreshadowing? ApoE4 Disrupts Brain Connectivity in Absence of Aβ
- Functional Connectivity Predicts Aβ Deposition in Mice
Research Models Citations
Mutations Citations
Paper Citations
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001 Jan 16;98(2):676-82. PubMed.
- Hedden T, Van Dijk KR, Becker JA, Mehta A, Sperling RA, Johnson KA, Buckner RL. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci. 2009 Oct 7;29(40):12686-94. PubMed.
- Liang Z, King J, Zhang N. Uncovering intrinsic connectional architecture of functional networks in awake rat brain. J Neurosci. 2011 Mar 9;31(10):3776-83. PubMed.
- Ahrens ET, Dubowitz DJ. Peripheral somatosensory fMRI in mouse at 11.7 T. NMR Biomed. 2001 Aug;14(5):318-24. PubMed.
- Nair G, Duong TQ. Echo-planar BOLD fMRI of mice on a narrow-bore 9.4 T magnet. Magn Reson Med. 2004 Aug;52(2):430-4. PubMed.
- Adamczak JM, Farr TD, Seehafer JU, Kalthoff D, Hoehn M. High field BOLD response to forepaw stimulation in the mouse. Neuroimage. 2010 Jun;51(2):704-12. Epub 2010 Mar 6 PubMed.
- Jonckers E, Van Audekerke J, De Visscher G, Van der Linden A, Verhoye M. Functional connectivity fMRI of the rodent brain: comparison of functional connectivity networks in rat and mouse. PLoS One. 2011 Apr 18;6(4):e18876. PubMed.
- Shah D, Jonckers E, Praet J, Vanhoutte G, Delgado Y Palacios R, Bigot C, D'Souza DV, Verhoye M, Van der Linden A. Resting state FMRI reveals diminished functional connectivity in a mouse model of amyloidosis. PLoS One. 2013;8(12):e84241. Epub 2013 Dec 17 PubMed.
- Grandjean J, Schroeter A, Batata I, Rudin M. Optimization of anesthesia protocol for resting-state fMRI in mice based on differential effects of anesthetics on functional connectivity patterns. Neuroimage. 2014 Aug 28;102P2:838-847. PubMed.
- Umeda T, Tomiyama T, Kitajima E, Idomoto T, Nomura S, Lambert MP, Klein WL, Mori H. Hypercholesterolemia accelerates intraneuronal accumulation of Aβ oligomers resulting in memory impairment in Alzheimer's disease model mice. Life Sci. 2012 Jan 17; PubMed.
- Berezovska O, Jack C, Deng A, Gastineau N, Rebeck GW, Hyman BT. Notch1 and amyloid precursor protein are competitive substrates for presenilin1-dependent gamma-secretase cleavage. J Biol Chem. 2001 Aug 10;276(32):30018-23. Epub 2001 Jun 14 PubMed.
- Allemang-Grand R, Scholz J, Ellegood J, Cahill LS, Laliberté C, Fraser PE, Josselyn SA, Sled JG, Lerch JP. Altered brain development in an early-onset murine model of Alzheimer's disease. Neurobiol Aging. 2015 Feb;36(2):638-47. Epub 2014 Sep 6 PubMed.
External Citations
Further Reading
Papers
- Sheline YI, Raichle ME. Resting State Functional Connectivity in Preclinical Alzheimer's Disease. Biol Psychiatry. 2013 Jan 3; PubMed.
Primary Papers
- Grandjean J, Schroeter A, He P, Tanadini M, Keist R, Krstic D, Konietzko U, Klohs J, Nitsch RM, Rudin M. Early alterations in functional connectivity and white matter structure in a transgenic mouse model of cerebral amyloidosis. J Neurosci. 2014 Oct 8;34(41):13780-9. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Bio-Imaging Lab, University of Antwerp
Grandjean and colleagues used resting-state functional MRI (rsfMRI) techniques in ArcAβ and wild-type mice at different time points across their lifespan to investigate the relation between functional connectivity disruptions and amyloid pathology. They observed compromised development of functional connectivity between sensory-motor regions during the first months of postnatal life along with structural changes in the brain.
In a previous study at the Bio-Imaging Lab, we demonstrated that functional connectivity disruptions could be observed in the APP/PS1 mouse model of amyloidosis (Shah et al., 2013). The APP/PS1 mice show a rapid onset and progression of amyloid pathology, with the first amyloid plaques appearing around 6-8 weeks of age in the cortex (Radde et al., 2006). In our study, the APP/PS1 and wild-type mice were investigated with rsfMRI at the age of 18-19 months, when amyloid plaques are extensively present throughout the entire brain. The next logical step after this proof-of-concept study was to investigate functional connectivity alterations at earlier stages and even before the onset of amyloid plaque development. The rationale behind this idea is the fact that soluble forms of Aβ are known to exert toxic effects at the level of the synapses (Mucke and Selkoe, 2012), thus it would seem plausible to observe functional changes before amyloid plaques even develop. This is further supported by the observation of cognitive dysfunctions before the onset of plaque development in some mouse models of amyloidosis (Dodart et al., 1999; Knobloch et al., 2007; Westerman et al., 2002).
In line with this idea, the most promising result of the study of Grandjean and colleagues is that functional connectivity disruptions could already be observed before the onset of amyloid plaques. The ArcAβ mice develop intracellular punctate deposits of Aβ at the age of 6 months, but overt amyloid plaque formation and cerebral amyloid angiopathy (CAA), i.e. deposits of amyloid in the vessel walls, only develop between 9-15 months of age. The first cognitive deficits, however, start at 6 months of age (Knobloch et al., 2007), strongly suggesting that stages earlier than overt amyloid plaque formation might be the cause of the observed memory disruptions. The early functional connectivity deficits were observed mainly in sensory-motor regions, suggesting that these regions are the most vulnerable to amyloid pathology. This is in contrast with findings in Alzheimer’s disease patients where the default-mode-network (DMN) seems to be the most vulnerable to AD pathology. Grandjean and colleagues explain this discrepancy by the DMN being the metabolically most active network in humans and the sensory regions being metabolically as demanding as the cingulate regions in rodents. We strongly believe that conclusions of any rsfMRI results must be made taking into account the characteristics of the mouse model and the anesthesia regime that is used. Therefore, studies in different mouse models of amyloidosis with different patterns of disease progression and the use of different anesthesia regimes might provide even more insight into which brain regions are specifically vulnerable and how this can be related to amyloid pathology.
Resting-state functional MRI has proven to be a nice technique to detect functional changes in humans and rats. Recently, more and more resting-state functional MRI studies are also being performed in mice and mouse models of disease. The main advantages of this technique are its non-invasive character and short acquisition times, rendering it favorable in terms of translation to the clinic. Considering the existence of numerous interesting mouse models of disease, it is of great importance to optimize this technique in healthy mice and mouse models. This study by Grandjean and colleagues nicely shows how resting-state functional MRI can contribute to identifying early stage functional alterations in mouse models of disease.
References:
Dodart JC, Meziane H, Mathis C, Bales KR, Paul SM, Ungerer A. Behavioral disturbances in transgenic mice overexpressing the V717F beta-amyloid precursor protein. Behav Neurosci. 1999 Oct;113(5):982-90. PubMed.
Knobloch M, Konietzko U, Krebs DC, Nitsch RM. Intracellular Abeta and cognitive deficits precede beta-amyloid deposition in transgenic arcAbeta mice. Neurobiol Aging. 2007 Sep;28(9):1297-306. Epub 2006 Jul 31 PubMed.
Mucke L, Selkoe DJ. Neurotoxicity of Amyloid β-Protein: Synaptic and Network Dysfunction. Cold Spring Harb Perspect Med. 2012 Jul;2(7):a006338. PubMed.
Radde R, Bolmont T, Kaeser SA, Coomaraswamy J, Lindau D, Stoltze L, Calhoun ME, Jäggi F, Wolburg H, Gengler S, Haass C, Ghetti B, Czech C, Hölscher C, Mathews PM, Jucker M. Abeta42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep. 2006 Sep;7(9):940-6. PubMed.
Shah D, Jonckers E, Praet J, Vanhoutte G, Delgado Y Palacios R, Bigot C, D'Souza DV, Verhoye M, Van der Linden A. Resting state FMRI reveals diminished functional connectivity in a mouse model of amyloidosis. PLoS One. 2013;8(12):e84241. Epub 2013 Dec 17 PubMed.
Westerman MA, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin LH, Carlson GA, Younkin SG, Ashe KH. The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer's disease. J Neurosci. 2002 Mar 1;22(5):1858-67. PubMed.
View all comments by Disha ShahMake a Comment
To make a comment you must login or register.