In Mice, TREM2 Antibody Mobilizes Microglia, Yet Worsens Tangles
Quick Links
In the October 11 Journal of Experimental Medicine, scientists led by David Holtzman, Washington University School of Medicine in St. Louis, reported that a TREM2 agonistic antibody mobilized microglia to surround plaques, yet did not abate amyloid load. Furthermore, the antibody exacerbated tau seeding, the spread of neurofibrillary tangles, and the formation of dystrophic neurites. The authors caution that, should TREM2 activation not robustly clear amyloid plaques, it could make AD neuropathology worse. Several TREM2 antibodies are being developed to treat AD, and one is in a Phase 2 trial.
Marco Colonna and Yingyue Zhou of WashU thought these results were interesting but difficult to explain. “It is unclear how Aβ accumulation is linked to tau aggregation, not to mention the role of TREM2 or microglia in this process,” they wrote. Jacob George, Kaplan Medical Center, Rehovot, Israel, agreed. “This important study points to the complexity of TREM2’s interaction with the various determinants of neurodegeneration,” he wrote (comments below).
Loss-of-function variants in the microglial receptor TREM2 increase a person’s risk of developing sporadic Alzheimer’s disease. Faulty TREM2 signaling hobbles microglial response to Aβ, which worsens amyloid plaques, tau tangles, and neurodegeneration (Jul 2019 conference news; May 2016 news). Would boosting TREM2 signaling reduce plaques and prevent neurodegeneration? The answer hasn’t been straightforward.
Scientists led by Christian Haas at Ludwig Maximilians University, Munich, developed an antibody that activates TREM2. In mice, it reduced amyloid load and improved memory (Mar 2020 news). Haass collaborates with scientists at Denali Therapeutics, South San Francisco, to develop this antibody for clinical testing. Similarly, scientists led by Zhiqiang An and Ningyan Zhang at the University of Texas Health Science Center, Houston, created a tetravalent anti-TREM2 antibody that mobilized microglia and reduced plaque load in mice (Sep 2022 news). Donna Wilcock of the University of Kentucky, Lexington, found much the same in mice given the TREM2 agonist antibody AL002a (May 2019 conference news; Dec 2016 conference news). This is the antibody Holtzman and colleagues used for their study.
However, scientists led by Colonna and Tina Schwabe at Alector LLC, South San Francisco, reported that AL002c, which binds the same epitope, had no effect on plaque load or microglial numbers, but did reduce neurodegeneration and preserved memory in mice expressing human TREM2 (Jun 2020 news). Alector is testing AL002c in an ongoing Phase 2 trial in early AD. To complicate matters further, scientists led by Timothy Miller at WashU found that suppressing TREM2 expression lowered plaques in mice, questioning whether activation or silencing this microglial receptor would be the best therapeutic approach (Jul 2021 news).
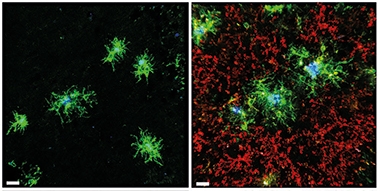
DAM Boost. Brain tissue from 5xFAD mice given the TREM2 agonist AL002a (right) have more DAMs (green) swarming amyloid plaques (blue), yet the same number of homeostatic microglia (red) as untreated 5xFAD mice (left). [Courtesy of Jain et al., Journal of Experimental Medicine, 2022.]
Now, the findings from Holtzman’s group add to the unease. Curious about how TREM2 activation influences tau seeding, first author Nimansha Jain injected AL002a into the abdomens of 6-month-old 5xFAD mice. These animals have extensive plaques and gliosis, the beginnings of neurodegeneration, and impaired memory. However, they do not develop tangles. To kick-start that process, Jain injected tau aggregates from AD brain tissue into the mice's hippocampi and cortices one week after they had started AL002a.
The antibody treatment boosted microglial numbers by half in the hippocampus and cortex. Around plaques, twice as many microglia expressed disease-associated microglia (DAM) markers CLEC7A, CD68, or APOE as in untreated mice (Jun 2017 news). The authors concluded that the antibody increased TREM2 signaling and the DAM response (see image above).
However, total and fibrillar plaque load, as measured by the antibody HJ3.4 and the dye X34, respectively, were similar in treated and control mice. This suggests that while AL002a recruited more microglia to plaques, the cells did not clear Aβ or compact the aggregates.
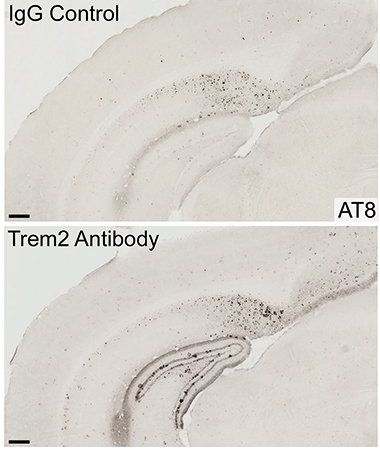
More TREM2 Signaling, More Tangles. Brain tissue from AL002a-treated 5xFAD mice (bottom) had more phospho-tau deposits (black) than did tissue from untreated controls (top). [Courtesy of Jain et al., Journal of Experimental Medicine, 2022.]
What about tau? Unexpectedly, the antibody accelerated tau seeding. It increased AT8-positive phospho-tau surrounding plaques fourfold (see image at left). Treated mice also had 1.4-times as many swollen dystrophic neurites and 25 percent fewer synapses than did untreated controls.
The stable amyloid load and worsened tau pathology surprised the authors. since they had found previously that seeded tau pathology was worse in APP/PS1 mice when TREM2 was knocked out from birth (Leyns et al., 2019). They attributed the same effect in the antibody-treated mice to the timing of TREM2 activation. “Results from this study emphasize the fact that microglial function is context- and disease-stage-dependent,” the authors wrote.
In other words, TREM2 signaling could be either protective or harmful, depending on the type and extent of AD pathology. “Our data suggest that sustained microglial activation through TREM2 that does not result in strong amyloid removal may exacerbate Aβ-induced tau pathology, which may have important clinical implications,” the authors concluded. George agreed. “This research supports the rationale of using TREM2 immunotherapy at earlier stages of AD when Aβ load is negligible and thus less influential as a driver of tau pathology,” he wrote.—Chelsea Weidman Burke
References
Mutation Interactive Images Citations
News Citations
- TREM2, Microglia Dampen Dangerous Liaisons Between Aβ and Tau
- Barrier Function: TREM2 Helps Microglia to Compact Amyloid Plaques
- Paper Alert: Mouse TREM2 Antibody Boosts Microglial Plaque Clean-Up
- Potent TREM2 Antibody Stirs Microglia to Prune Plaques in Mice
- Antibodies Against Microglial Receptors TREM2 and CD33 Head to Trials
- Inflammation Helps Microglia Clear Amyloid from AD Brains
- In Mice, Activating TREM2 Tempers Plaque Toxicity, not Load
- New Ways to Target TREM2 Beg the Question: Up or Down?
- Hot DAM: Specific Microglia Engulf Plaques
Therapeutics Citations
Research Models Citations
Paper Citations
- Leyns CE, Gratuze M, Narasimhan S, Jain N, Koscal LJ, Jiang H, Manis M, Colonna M, Lee VM, Ulrich JD, Holtzman DM. TREM2 function impedes tau seeding in neuritic plaques. Nat Neurosci. 2019 Aug;22(8):1217-1222. Epub 2019 Jun 24 PubMed.
Further Reading
Papers
- Fassler M, Rappaport MS, Cuño CB, George J. Engagement of TREM2 by a novel monoclonal antibody induces activation of microglia and improves cognitive function in Alzheimer's disease models. J Neuroinflammation. 2021 Jan 9;18(1):19. PubMed.
Primary Papers
- Jain N, Lewis CA, Ulrich JD, Holtzman DM. Chronic TREM2 activation exacerbates Aβ-associated tau seeding and spreading. J Exp Med. 2023 Jan 2;220(1) Epub 2022 Oct 11 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Cognyxx Pharmaceuticals
The paper by Jain et al. adds an important piece to the complex puzzle of the interaction of TREM2 with microglia in the context of Aβ-associated tau seeding.
The authors found, apparently counterintuitively, that TREM2 chronic agonism with a monoclonal activating antibody, previously shown to reduce Aβ-related pathology (Wang et al., 2020), led to an increase in tau seeding in 5xFAD mice injected with human-derived tau. This finding was corroborated by validation of downstream “biological” target engagement of the antibody, by observing classic TREM2-related Syk phosphorylation and microglial transformation to a DAM phenotype. This finding is particularly interesting as the human surrogate of this antibody is being tested in a Phase 2 clinical trial in AD, and secondary endpoints in the CSF will be looked at to see how the observations put forward herein would be translatable (see clinicaltrials.gov).
The complex interaction of TREM2 expressed in microglia and Aβ-related pathology has been explored in several animal models and the complete absence of this membrane receptor or its impaired function have been associated with a lack of appropriate plaque coverage by microglia. TREM2 agonism has been tested with several monoclonal antibodies given at different time points with regard to the initiation of Aβ-mediated pathology, and typically demonstrated better plaque coverage by microglia and reduction of size and composition of the plaque (Wang et al., 2020; Price et al., 2020; Schlepckow et al., 2020; Fassler et al., 2021). There have been differences in the design of the studies with regard to the dosages and timing of the initiation of treatment and no data on TREM2 receptor occupancy to allow an accurate comparison. Additionally, the pharmacokinetics/pharmacodynamics has not been reported for all studies, so the differences cannot be analyzed comparatively.
With regard to the interaction of tau and TREM2, studies prior to the current one tested the effects of gene deletion or impaired function on tau pathology driven by Aβ, showing that the presence of TREM2 limited tau expression, yet no TREM2 activation approach was yet tested. In this paper, the investigators used a model where tau injection was performed after Aβ was already present and tested the effects of chronic TREM2 activation by the antibody. A major advantage here is the ability to exactly time tau-inducible pathology, which may be more relevant to human AD. Indeed, this important study points to the complexity of the interaction of TREM2 with the various determinants of neurodegeneration and adds further support to the rationale of using the immunotherapy at earlier stages of AD when Aβ load is negligible and thus less influential as a driver of tau pathology.
However, several points should be taken into consideration when trying to extrapolate from the murine data to future implementation of the antibody:
Regardless of the above points, the paper by Jain et al. adds important information with regard to the potential therapeutic window of TREM2 immunotherapy, and it emphasizes the need to gain more insight into the role of this membrane receptor in the pathogenesis of AD. TREM2 remains an exciting target to address therapeutically, given the strong genetic-based studies and the indifference of the approach with regard to the nature of the protein aggregate triggering neurodegeneration in AD patients.
References:
Wang S, Mustafa M, Yuede CM, Salazar SV, Kong P, Long H, Ward M, Siddiqui O, Paul R, Gilfillan S, Ibrahim A, Rhinn H, Tassi I, Rosenthal A, Schwabe T, Colonna M. Anti-human TREM2 induces microglia proliferation and reduces pathology in an Alzheimer's disease model. J Exp Med. 2020 Sep 7;217(9) PubMed.
Price BR, Sudduth TL, Weekman EM, Johnson S, Hawthorne D, Woolums A, Wilcock DM. Therapeutic Trem2 activation ameliorates amyloid-beta deposition and improves cognition in the 5XFAD model of amyloid deposition. J Neuroinflammation. 2020 Aug 14;17(1):238. PubMed.
Schlepckow K, Monroe KM, Kleinberger G, Cantuti-Castelvetri L, Parhizkar S, Xia D, Willem M, Werner G, Pettkus N, Brunner B, Sülzen A, Nuscher B, Hampel H, Xiang X, Feederle R, Tahirovic S, Park JI, Prorok R, Mahon C, Liang CC, Shi J, Kim DJ, Sabelström H, Huang F, Di Paolo G, Simons M, Lewcock JW, Haass C. Enhancing protective microglial activities with a dual function TREM2 antibody to the stalk region. EMBO Mol Med. 2020 Apr 7;12(4):e11227. Epub 2020 Mar 10 PubMed.
Fassler M, Rappaport MS, Cuño CB, George J. Engagement of TREM2 by a novel monoclonal antibody induces activation of microglia and improves cognitive function in Alzheimer's disease models. J Neuroinflammation. 2021 Jan 9;18(1):19. PubMed.
Washington University in St. Louis
Washington University School of Medicine
Jain et al. report surprising results that chronically activating TREM2 after plaque formation increases neuritic plaque (NP)-tau pathology and Aβ-associated tau spreading when 5XFAD mice brains are injected with human tau aggregates. The results showed, undoubtedly, that TREM2 activation through an agonistic antibody boosts microglia toward a DAM-like state, consistent with previous reports. However, this boost did not result in amelioration of plaque or NP-tau pathology, re-proposing the question as to whether TREM2 therapeutics are relevant to amyloidosis or tau pathology.
It is yet unclear how Aβ accumulation is linked to tau aggregation, not to mention the role of TREM2, or microglia, in this process. The authors showed that acute TREM2 activation did not affect AD-tau phagocytosis in bone-marrow-derived macrophages. Whether chronic TREM2 activation increases microglia phagocytic activity remains an area of investigation. More mechanistic studies are needed before conclusions can be made on the timing of TREM2 immunotherapy. Nonetheless, studies like Jain et al. are important as we accumulate evidence on the potential effects of TREM2 agonists/antagonists on AD pathology.
Alector
Alector
Lundbeck
The results from Dr. Holtzman’s group add to a growing literature on the complex, confusing, and often contradictory reported effects of TREM2 on amyloid and tau pathology in animal models.
For example, Dr. Holtzman’s group had previously shown that crossing TREM2 knockout mice with PS19 tau transgenic mice resulted in less brain atrophy but without affecting phosphorylated or insoluble tau (Leyns et al., 2017), supporting a detrimental effect of TREM2 on AD pathology. However, the Holtzman lab also reported that in both a mouse model of tau seeding and in AD patients, loss of TREM2 function facilitated seeding and spreading of neuritic plaque tau aggregates (Leyns et al., 2019) supporting a beneficial effect of TREM2 on tau pathology.
Other labs have reported that TREM2 knockout mice crossed to APP/PS1 mice resulted in decreased amyloid and tau pathology (Jay et al., 2015) and that acute antisense oligonucleotide-mediated knockdown of TREM2 led to reduced plaque deposition and pathology and to a reduced phosphorylated tau expression in 11-month-old APP/PS1 mice (Schoch et al., 2021), arguing for a detrimental effect of TREM2 on both amyloid and tau pathology.
In addition, multiple independent studies support the idea that TREM2 is neuroprotective against amyloid and tau pathology. For example, crossing TREM2 knockout mice to hTau mice was shown to accelerate and exacerbate hyperphosphorylation and aggregation of tau and dysregulate neuronal stress kinase pathways (Bemiller et al., 2017). Likewise, crossing TREM2 knockout mice with 5XFAD mice was reported to elevate Aβ accumulation (Wang et al., 2015). Crossing TREM2 knockout mice with TauPS2APP mice, which contain both amyloid and tau pathology, further exacerbated tau accumulation and spreading and promoted brain atrophy (Lee et al., 2021). In addition, different antibodies that activate TREM2 have been shown to reduce amyloidogenesis in 6-month-old APP knock-in mice and reduce inflammation, filamentous plaques, neuritic dystrophy, and behavioral impairment in 8-month-old 5XFAD mice (Schlepckow et al., 2020; Wang et al., 2020).
The conflicting animal data highlights the complexity associated with reproducing a human-specific disease in rodents, and our partial understanding of Alzheimer's disease pathology. It remains to be seen why these animal results are disparate: Is it the model (amyloid, tau, or both), the treatment regimen, the timing of treatment, the difference between genetic ablation and pharmacological intervention, or combinations of all the above? Additional experiments will lead to a better understanding of the interplay between TREM2, microglial activation, and AD pathology.
Unlike the animal models which convey lab-dependent, model-dependent, and context-dependent outcomes, the human genetics for TREM2 appear crystal clear. Complete loss of TREM2 function, or its signaling co-receptor DAP12, invariably lead to early onset forms of dementia (Paloneva et al., 2002; Paloneva et al., 2000). Likewise, heterozygous loss-of-function point mutations in TREM2, which lead to decreased ability to bind TREM2 ligands, lead to increased risk of developing AD (Guerreiro et al., 2013; Jonsson et al., 2013). These data suggest that activation of TREM2 could be beneficial for AD, and likely other neurodegenerative disorders. To test this hypothesis, Alector is currently testing its activating TREM2 antibody, AL002, in a double blinded Phase 2 study in AD patients.
References:
Leyns CE, Ulrich JD, Finn MB, Stewart FR, Koscal LJ, Remolina Serrano J, Robinson GO, Anderson E, Colonna M, Holtzman DM. TREM2 deficiency attenuates neuroinflammation and protects against neurodegeneration in a mouse model of tauopathy. Proc Natl Acad Sci U S A. 2017 Oct 24;114(43):11524-11529. Epub 2017 Oct 9 PubMed.
Leyns CE, Gratuze M, Narasimhan S, Jain N, Koscal LJ, Jiang H, Manis M, Colonna M, Lee VM, Ulrich JD, Holtzman DM. TREM2 function impedes tau seeding in neuritic plaques. Nat Neurosci. 2019 Aug;22(8):1217-1222. Epub 2019 Jun 24 PubMed.
Jay TR, Miller CM, Cheng PJ, Graham LC, Bemiller S, Broihier ML, Xu G, Margevicius D, Karlo JC, Sousa GL, Cotleur AC, Butovsky O, Bekris L, Staugaitis SM, Leverenz JB, Pimplikar SW, Landreth GE, Howell GR, Ransohoff RM, Lamb BT. TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer's disease mouse models. J Exp Med. 2015 Mar 9;212(3):287-95. Epub 2015 Mar 2 PubMed.
Schoch KM, Ezerskiy LA, Morhaus MM, Bannon RN, Sauerbeck AD, Shabsovich M, Jafar-Nejad P, Rigo F, Miller TM. Acute Trem2 reduction triggers increased microglial phagocytosis, slowing amyloid deposition in mice. Proc Natl Acad Sci U S A. 2021 Jul 6;118(27) PubMed.
Bemiller SM, McCray TJ, Allan K, Formica SV, Xu G, Wilson G, Kokiko-Cochran ON, Crish SD, Lasagna-Reeves CA, Ransohoff RM, Landreth GE, Lamb BT. TREM2 deficiency exacerbates tau pathology through dysregulated kinase signaling in a mouse model of tauopathy. Mol Neurodegener. 2017 Oct 16;12(1):74. PubMed.
Wang Y, Cella M, Mallinson K, Ulrich JD, Young KL, Robinette ML, Gilfillan S, Krishnan GM, Sudhakar S, Zinselmeyer BH, Holtzman DM, Cirrito JR, Colonna M. TREM2 lipid sensing sustains the microglial response in an Alzheimer's disease model. Cell. 2015 Mar 12;160(6):1061-71. Epub 2015 Feb 26 PubMed.
Lee SH, Meilandt WJ, Xie L, Gandham VD, Ngu H, Barck KH, Rezzonico MG, Imperio J, Lalehzadeh G, Huntley MA, Stark KL, Foreman O, Carano RA, Friedman BA, Sheng M, Easton A, Bohlen CJ, Hansen DV. Trem2 restrains the enhancement of tau accumulation and neurodegeneration by β-amyloid pathology. Neuron. 2021 Apr 21;109(8):1283-1301.e6. Epub 2021 Mar 5 PubMed.
Schlepckow K, Monroe KM, Kleinberger G, Cantuti-Castelvetri L, Parhizkar S, Xia D, Willem M, Werner G, Pettkus N, Brunner B, Sülzen A, Nuscher B, Hampel H, Xiang X, Feederle R, Tahirovic S, Park JI, Prorok R, Mahon C, Liang CC, Shi J, Kim DJ, Sabelström H, Huang F, Di Paolo G, Simons M, Lewcock JW, Haass C. Enhancing protective microglial activities with a dual function TREM2 antibody to the stalk region. EMBO Mol Med. 2020 Apr 7;12(4):e11227. Epub 2020 Mar 10 PubMed.
Wang S, Mustafa M, Yuede CM, Salazar SV, Kong P, Long H, Ward M, Siddiqui O, Paul R, Gilfillan S, Ibrahim A, Rhinn H, Tassi I, Rosenthal A, Schwabe T, Colonna M. Anti-human TREM2 induces microglia proliferation and reduces pathology in an Alzheimer's disease model. J Exp Med. 2020 Sep 7;217(9) PubMed.
Paloneva J, Manninen T, Christman G, Hovanes K, Mandelin J, Adolfsson R, Bianchin M, Bird T, Miranda R, Salmaggi A, Tranebjaerg L, Konttinen Y, Peltonen L. Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am J Hum Genet. 2002 Sep;71(3):656-62. Epub 2002 Jun 21 PubMed.
Paloneva J, Kestilä M, Wu J, Salminen A, Böhling T, Ruotsalainen V, Hakola P, Bakker AB, Phillips JH, Pekkarinen P, Lanier LL, Timonen T, Peltonen L. Loss-of-function mutations in TYROBP (DAP12) result in a presenile dementia with bone cysts. Nat Genet. 2000 Jul;25(3):357-61. PubMed.
Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JS, Younkin S, Hazrati L, Collinge J, Pocock J, Lashley T, Williams J, Lambert JC, Amouyel P, Goate A, Rademakers R, Morgan K, Powell J, St George-Hyslop P, Singleton A, Hardy J, Alzheimer Genetic Analysis Group. TREM2 variants in Alzheimer's disease. N Engl J Med. 2013 Jan 10;368(2):117-27. Epub 2012 Nov 14 PubMed.
Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, Rujescu D, Hampel H, Giegling I, Andreassen OA, Engedal K, Ulstein I, Djurovic S, Ibrahim-Verbaas C, Hofman A, Ikram MA, van Duijn CM, Thorsteinsdottir U, Kong A, Stefansson K. Variant of TREM2 associated with the risk of Alzheimer's disease. N Engl J Med. 2013 Jan 10;368(2):107-16. Epub 2012 Nov 14 PubMed.
Mayo Clinic Florida
This paper clearly shows that DAM or neurodegenerative microglia (MGnD) induction accelerates tau propagation in 5xFAD mice. This is consistent with our recent report showing accelerated tau propagation in a humanized APP NL-G-F knock-in mice, which is sensitive to microglial depletion.
We show that this is associated with enhanced secretion of extracellular vesicles (EV) containing tau from activated microglia (Clayton et al., 2021), suggesting a role for EV as the mediator for tau propagation via microglial activation. TREM2 antibody therapy thus needs to be carefully designed to avoid worsening of tau pathology via microglial activation.
References:
Clayton K, Delpech JC, Herron S, Iwahara N, Ericsson M, Saito T, Saido TC, Ikezu S, Ikezu T. Plaque associated microglia hyper-secrete extracellular vesicles and accelerate tau propagation in a humanized APP mouse model. Mol Neurodegener. 2021 Mar 22;16(1):18. PubMed. Correction.
TrueBinding
This is an interesting paper reproducing the interaction of a TREM2 antibody with microglia. Unfortunately this TREM2 antibody (AL002a ) doesn't show significant reduction in amyloid plaque load in 5xFAD mice. Injecting tau aggregates from AD brain tissue into these mice exacerbates tau pathology.
I am wondering if a similar enhancement in tau pathology or neurofibrillary tangles occurs by injecting tau seeds into in tau transgenic mice (P301L, P301S), or any other AD transgenic mice containing tau mutations, such as 3xTg mice. It is already known that Aβ acts as a seed for induction of tau pathology. This shows that this TREM-2 antibody doesn't reduce microglia activation. This, in turn, doesn't improve the phagocytic activity of microglia to remove amyloid plaques, which leads to more aggregation of tau. It will be interesting to investigate whether different conformational tau aggregates lead to similar effect.
Make a Comment
To make a comment you must login or register.