New Rodent Knock-Ins Carry Humanized Wild-Type APP
Quick Links
Creating better, more reliable rodent models of Alzheimer’s disease has been a struggle for researchers. On October 19 in Molecular Neurodegeneration, scientists led by Bart De Strooper at KU Leuven, Belgium, debuted new mouse and rat knock-in models that express a humanized form of the wild-type amyloid precursor protein. Mouse β-secretase (BACE) cleaves this APP three times faster than it cleaves the endogenous rodent form, putting cleavage on par with that in the human brain. The new models produce Aβ peptides that more closely match their human homologs, and that can be detected by highly sensitive human Aβ ELISAs. De Strooper believes these new models will serve as better controls for knock-ins that express human APP carrying familial AD mutations.
- Knock-ins have more soluble Aβ in their brains than wild-types, but half as much as in human brain.
- The humanized Aβ peptides can be detected by highly sensitive ELISAs.
- APP/PSEN1 knock-in rats showed no Aβ plaques up to 2 years old.
Many mouse models of AD overexpress mutant human APP and/or presenilin. While they are reasonable models of amyloidosis, and sometimes gliosis, most lack the hallmark neuronal loss seen in people with Alzheimer’s. Additionally, some scientists believe that the pathologies may be artifacts of protein overexpression or of the random insertion of transgenes into the mouse genome (Apr 2014 webinar; Sep 2016 news).
Takaomi Saido and colleagues at the RIKEN Brain Science Institute in Wako, Japan, skirted the aberrant expression problem by knocking mutant forms of human APP into the mouse APP locus (Saito et al., 2014). They made mice expressing APP with combinations of the Swedish (KM670/671NL), Iberian (I716F), and Arctic (E693G) mutations. Their NL-F and NL-G-F knock-ins make more Aβ than do wild-type mice, accumulate plaques in the brain, have obvious gliosis, and perform poorly in cognitive tests (Saito et al., 2014).
“Saido demonstrated the crucial importance of using knock-in mutations in the APP gene instead of the classical minigenes to study the effect of AD mutations in APP,” De Strooper wrote to Alzforum. “He also humanized the three amino acids that distinguish the mouse and human Aβ sequence in APP, which we had shown many years ago affect BACE processing and overall amounts of Aβ” (De Strooper, et al., 1995).
However, Saido’s group created no control mouse with humanized wild-type APP. How much of the NL-F and NL-G-F phenotypes are due to the Swedish, Iberian, and Arctic mutations rather than the humanized wild-type substitutions? To answer this question, first author Lutgarde Serneels and colleagues in De Strooper’s lab used CRISPR/Cas9 to edit mouse and rat APP to convert the G676, F681, and R684 residues into their respective R, Y, and H human variants, leaving the rest of the APP gene and its expression patterns unchanged.
The knock-in rat adds to the small yet growing list of rat AD models and provides a starting point for testing various other mutations, such as presenilin 1 M139T, as the authors have done here. Recently, Luciano D’Adamio’s lab at Rutgers University made a similar knock-in rat model containing the same three human APP changes (Tambini et al., 2019).
The new humanized APP rodents, called APPhu/hu, expressed similar levels of APP in the brain as did wild-type rodents. However, the APPhu/hu mice and rats had 2.5- and 4.2-fold more β-C-terminal fragments (β-CTF) and 0.6- and 0.7-fold lower α-CTFs than wild-type mice and rats, respectively. This makes sense given that mouse BACE more efficiently cleaves the humanized APP. Serneels noted that the APPhu/hu knock-in mice share the same C57BL/6 background as Saido’s mutant APP knock-ins, making them ideal controls.
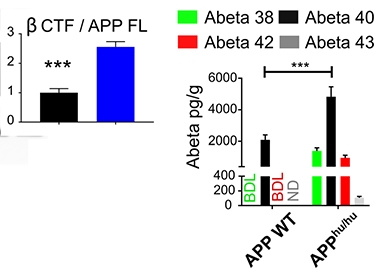
Aβ Changes. APPhu/hu mice make more β-CTF (left) than do wild-type mice. They also produce more Aβ peptides (right). [Courtesy of Serneels et al., 2020, Mol Neurodegen.]
What about Aβ? APPhu/hu mice made 2.3-fold more Aβ40 than did wild-type mice, while APPhu/hu rats made about three times as much as did wild-type controls (see image at right). Though Aβ38, Aβ42, and Aβ43 could not be detected in the wild-type rodents, they were detected in APPhu/hu animals. Total soluble Aβ levels in the knock-in brains were higher than in wild-type, but still only about half the levels found in tissue removed from the human brain during surgery for epilepsy.
The researchers also created APPhu/hu rats with a homozygous M139T familial AD mutation in their presenilin genes. People carrying this variant begin to show symptoms of dementia by their mid- to late 40s.
Similar to the APPhu/hu knock-in, the APPhu/hu/PSEN1 knock-in rats had about fourfold more β-CTF in the brain than did wild-type rats. However, while the total soluble Aβ levels in the APPhu/hu and APPhu/hu/PSEN1 knock-ins were the same, the PSEN1 mutants made more Aβ42, and less Aβ40 and Aβ43.
Despite this uptick in Aβ42, no amyloid plaques were found, even in 2-year-old APPhu/hu/PSEN1 rats. This seems to add weight to the idea that AD pathologies may be an artifact of transgene overexpression. However, Serneels said the knock-ins might need some additional pathology, such as a specific microglia response, to more closely mimic AD. Whether human and mouse microglia respond to plaques in the same way remains to be seen (Oct 2020 news; Jan 2020 news).
Further testing with the APPhu/hu knock-ins should reveal if humanized Aβ affects rodent behavior and cognition, noted Saido. “Comparison of De Strooper’s wild-type human Aβ line with the wild-type B6 line will clarify whether the human Aβ sequence causes any difference in behavior,” Saido wrote to Alzforum. “If there is no difference, just wild-type mice will be a good enough control.”
De Strooper is offering the knock-in mice for free and the two rat knock-in models for a nominal fee to defray development costs.—Chelsea Weidman Burke
References
Webinar Citations
News Citations
- Do APP Knock-ins Call Overexpression Models of AD into Question?
- Single-nucleus RNA Sequencing Misses Activation of Human Microglia
- Human and Mouse Microglia React Differently to Amyloid
Research Models Citations
- APP NL-F Knock-in
- APP NL-G-F Knock-in
- App knock-in (humanized Aβ)
- App knock-in (humanized Aβ)
- App knock-in (humanized Aβ) (Leuven)
- App knock-in (humanized Aβ) (Leuven); Psen1 knock-in (M139T)
Paper Citations
- Saito T, Matsuba Y, Mihira N, Takano J, Nilsson P, Itohara S, Iwata N, Saido TC. Single App knock-in mouse models of Alzheimer's disease. Nat Neurosci. 2014 May;17(5):661-3. Epub 2014 Apr 13 PubMed.
- De Strooper B, Simons M, Multhaup G, Van Leuven F, Beyreuther K, Dotti CG. Production of intracellular amyloid-containing fragments in hippocampal neurons expressing human amyloid precursor protein and protection against amyloidogenesis by subtle amino acid substitutions in the rodent sequence. EMBO J. 1995 Oct 16;14(20):4932-8. PubMed.
- Tambini MD, Yao W, D'Adamio L. Facilitation of glutamate, but not GABA, release in Familial Alzheimer's APP mutant Knock-in rats with increased β-cleavage of APP. Aging Cell. 2019 Dec;18(6):e13033. Epub 2019 Sep 9 PubMed.
Further Reading
Primary Papers
- Serneels L, T'Syen D, Perez-Benito L, Theys T, Holt MG, De Strooper B. Modeling the β-secretase cleavage site and humanizing amyloid-beta precursor protein in rat and mouse to study Alzheimer's disease. Mol Neurodegener. 2020 Oct 19;15(1):60. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.