Rethinking the Hippocampus
Quick Links
The hippocampus contains some of the best-studied neural circuits in the brain. It also plays a crucial role in learning and memory, and it is compromised in people with Alzheimer’s disease. How well do neuroscientists know their favorite seahorse? A trio of recent studies suggests that its connections and morphology are more complex than textbooks describe. In the September 2 Proceedings of the National Academy of Sciences, researchers report that axons run lengthwise along the hippocampus, forming a potentially important cadre of synapses—this in addition to the well-described transverse projections that researchers study in hippocampal slices. A paper in the August 31 Nature Neuroscience describes how hippocampal neurons transmit signals backward through those transverse projections. And in the September 17 Neuron, researchers report that a large number of hippocampal axons sprout directly from dendrites, rather than neuronal bodies. Together, the papers highlight features of the hippocampus that have flown under the radar.
“Understanding how the hippocampus encodes and relays information is fundamental to understanding memory,” said Cha-Min Tang, University of Maryland School of Medicine, Baltimore, the senior author on the PNAS paper.

Historically, hippocampal connections are thought to go from dentate gyrus to CA3, on to CA1 and then the subiculum, as pictured in this cross-section. Fewer connections are thought to join similar cell types of the same region (looking into the screen). [Image by Santiago Ramón y Cajal, Histologie du Système nerveux de l'Homme et des Vertébrés]
The prevailing notion about excitatory connections in the hippocampus is that they start in the dentate gyrus, project to the CA3, then on to CA1 and the subiculum (see image at left and Andersen et al., 1971). This “lamellar” hypothesis, which posits that neurons communicate between these areas and less so within them, led scientists to rely principally on cross-sectional or transverse slices for electrophysiological experiments of the hippocampus. These slices have been the mainstay for studying long-term potentiation, a form of synaptic plasticity that is essential for learning and memory. Researchers often use LTP to measure the effects of potentially toxic proteins and peptides, such as Aβ. Although scientists had previously noted that some axons run longitudinally through the CA3 and dentate gyrus, connecting to neighboring neurons in those regions (see image below), few studies had been done on those connections and they had generally been viewed as unimportant (see Amaral et al., 1991). Few of these types of axons had been observed in the CA1.
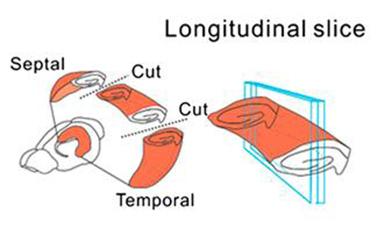
Scientists typically focus on connections within a hippocampal slice, depicted here by cross-sections made by the “cuts” through the hippocampus. Cha-Min Tang and colleagues at the University of Maryland School of Medicine focused on axons that extended through its longitudinal plane (blue rectangles). [Image courtesy of Yang et al., PNAS.]
Curious whether axons project within the CA1, scientists led by Tang studied intact hippocampi from C57BL/6 mice. First authors Sunggu Yang and Sungchil Yang, University of California, San Francisco, reported that axons from the CA1 neuron forked: One branch extended along the well-studied transverse axis to the subiculum, and the other longitudinally along the length of the CA1 region, projecting to other neurons there. Though the CA1 axons were thinner, they were present in every cell examined, leading Tang to believe that these projections do something important in the hippocampus.
Next, they found that the axons branching within the CA1 contacted pyramidal cell dendrites (see image below), and patch clamp recording from longitudinal hippocampal slices indicated that these synapses were functional. Further electrophysiological tests suggested that these excitatory connections strengthen in response to stimuli. Together, the findings imply that CA1 neurons in the hippocampus talk amongst themselves, the authors wrote.

Axons that branch within the CA1 (red) co-localize (yellow) with dendritic spines of CA1 pyramidal cells (green), suggesting they form working synapses. [Image courtesy of Yang et al., PNAS.]
The authors used high-powered techniques to demonstrate the existence of these connections, as well as reveal their anatomy, functionality, and plasticity,” said Howard Eichenbaum of Boston University. “This tells us there are powerful connections along the longitudinal axis, and we need to find out what those connections are doing.”
The longitudinal connections might explain how the hippocampus encodes temporal sequences, Tang said. “A chain of nearly identical neurons could transform a time sequence into a spatial one,” he told Alzforum. Such short-term “memory buffers” are essential for correctly interpreting information, such as the order of words in a sentence. The brain cannot function if it does not have such buffers to store the information in the correct temporal order, he said. Tang next plans to study how these connections work in animals receiving a sequence of inputs.
Raymond Kesner of the University of Utah, Salt Lake City, agreed. He has postulated that the CA1 puts a time stamp on memories, but had no explanation for how (see Hoge and Kesner, 2007). “[Tang] is providing a mechanism,” he told Alzforum. “This is impressive work.” Kesner pointed out that longitudinal projections do not refute the lamellar hypothesis; they simply add a way in which the CA1 contributes to hippocampal function.
The findings reinforce the point that “the hippocampus is three-dimensional,” Tang told Alzforum. “If you slice it, you lose those longitudinal connections.”
Another assumption about the hippocampus has to do with theta rhythms. These are waves of synaptic activity that course through the hippocampus at a frequency of about 4-7 Hz and are considered crucial for encoding and retrieving episodic memories (see Hasselmo et al., 2002). Since most anatomical and physiological studies of the hippocampus have focused on excitatory connections from CA3 to CA1 to the subiculum, researchers have presumed that theta rhythms reflect the same path. However, knowing that GABAergic interneurons are wired in the opposite direction (subiculum to dentate gyrus), scientists led by Sylvain Williams, McGill University, Montreal, tested whether theta activity might reflect signals travelling back to the dentate gyrus.
As described in Nature Neuroscience, first author Jesse Jackson and colleagues took intact hippocampi from Sprague-Dawley rats and simultaneously recorded spontaneous theta activity from the CA3, CA1, and subiculum. Both CA3 and subiculum generated their own theta rhythms. Surprisingly, the waves in the subiculum preceded those from CA3, hinting that the subiculum led to activation of CA3, not the other way around, as has been assumed. If the canonical model for neurotransmission in the hippocampus held true, CA3 activity should have led that of the subiculum, Williams said.
The authors found that GABAergic projections from the subiculum directly infiltrated the CA3, bypassing CA1. This suggests that subicular inhibitory neurons might suppress activity in the CA3. Furthermore, by manipulating the frequency of theta rhythms generated by the subiculum, the researchers changed those of CA3 in lock step.
To find out if such a back-projecting theta rhythm occurs in vivo, Jackson and colleagues monitored the same brain regions in freely moving rats and applied a mathematical technique called Granger causality to determine which way the activity flowed. They found that for much of the time, especially during REM sleep, theta rhythms flowed from the subiculum to CA3. GABAergic projections seemed to be involved in this flow, because the GABA(A) antagonist gabazine abolished the coordination between subiculum and CA3 in these rats.
Two decades ago, researchers including Gyorgy Buzsaki at New York University’s Langone Medical Center reported these back-projecting hippocampal inhibitory neurons (see Sik et al., 1994). “This paper is the first in 20 years to give some function to this anatomical observation,” Buzsaki told Alzforum. The implications could be far-reaching. “They describe an interesting mechanism that will probably modify our thinking about the hippocampus,” Buzsaki said. He pointed out that other coordinated neural activities, such as those that give rise to gamma waves (~40-100 Hz), also occur in the hippocampus, and wondered how they would be affected by these inhibitory projections.
“At the moment, it is hard to understand the functional significance of such a surprising finding,” Williams told Alzforum. “The subiculum, thought to be the main output of the hippocampus, could also be a very important input region,” he suggested. That could have implications for Alzheimer’s, he said. His group previously found irregularities in theta-gamma coupling in the subiculum of TgCRND8 mouse models of AD months before Aβ accumulation or memory loss (see Goutagny et al., 2013). “There is something going on in the subiculum very early in AD mouse models,” Williams said. “We need to understand the changes that occur in neural circuitry there; what types of cells are affected, and the underlying mechanisms.”
Like Tang’s study, Williams’ paper sheds light on hippocampal characteristics that depend on connections severed when researchers slice the structure in the transverse direction. “If you are interested in hippocampal activity, the best option is to use the whole hippocampal preparation, or a functioning hippocampus in vivo,” said Williams.

An Axon of a Different Color.
In a fluorescently labeled hippocampal neuron (red), an axon (white arrows) expressing the axon-specific marker ankyrin-G (green), branches off of a dendrite (black arrow). [Image courtesy of Neuron, Thome et al.]
The third study, reported in Neuron, suggests another anomaly in the hippocampus. Scientists led by Alexei Egorov, Heidelberg University, Germany, report in Neuron that instead of emanating from the soma as they normally do, axons in some pyramidal neurons of the hippocampus grow from dendrites (see image at left). Scientists had previously observed this characteristic in a small number of dendrites emerging from the apex of rat CA1 pyramidal cells (see Lorincz and Nusser, 2010), but first authors Christian Thome and Tony Kelly, who is at the University of Bonn, found that it is also common in dendrites at the base of these triangular cells. In mice, about half the neurons in the CA1 region had axons that originated on basal dendrites, as did more than a quarter of the cells in the CA3, and a fifth in the subiculum. Similar ratios turned up in a second mouse strain and in rats.
Dendrites containing axons were more excitable than dendrites without them, and their synaptic inputs were more likely to elicit action potentials—possibly because they are in such close proximity to the action potential "trigger zone" of the dendritic axon. These dendrites may represent a “privileged” channel for excitatory input into pyramidal cells, the authors wrote. They suggested next determining which excitatory inputs feed these dendrites.—Gwyneth Dickey Zakaib
References
Research Models Citations
Paper Citations
- Andersen P, Bliss TV, Skrede KK. Lamellar organization of hippocampal pathways. Exp Brain Res. 1971;13(2):222-38. PubMed.
- Amaral DG, Dolorfo C, Alvarez-Royo P. Organization of CA1 projections to the subiculum: a PHA-L analysis in the rat. Hippocampus. 1991 Oct;1(4):415-35. PubMed.
- Hoge J, Kesner RP. Role of CA3 and CA1 subregions of the dorsal hippocampus on temporal processing of objects. Neurobiol Learn Mem. 2007 Sep;88(2):225-31. Epub 2007 Jun 8 PubMed.
- Hasselmo ME, Bodelón C, Wyble BP. A proposed function for hippocampal theta rhythm: separate phases of encoding and retrieval enhance reversal of prior learning. Neural Comput. 2002 Apr;14(4):793-817. PubMed.
- Sik A, Ylinen A, Penttonen M, Buzsáki G. Inhibitory CA1-CA3-hilar region feedback in the hippocampus. Science. 1994 Sep 16;265(5179):1722-4. PubMed.
- Goutagny R, Gu N, Cavanagh C, Jackson J, Chabot JG, Quirion R, Krantic S, Williams S. Alterations in hippocampal network oscillations and theta-gamma coupling arise before Aβ overproduction in a mouse model of Alzheimer's disease. Eur J Neurosci. 2013 Jun;37(12):1896-902. PubMed.
- Lorincz A, Nusser Z. Molecular identity of dendritic voltage-gated sodium channels. Science. 2010 May 14;328(5980):906-9. PubMed.
Further Reading
Papers
- Nishida H, Takahashi M, Lauwereyns J. Within-session dynamics of theta-gamma coupling and high-frequency oscillations during spatial alternation in rat hippocampal area CA1. Cogn Neurodyn. 2014 Oct;8(5):363-72. Epub 2014 Apr 19 PubMed.
- Sweet JA, Eakin KC, Munyon CN, Miller JP. Improved learning and memory with theta-burst stimulation of the fornix in rat model of traumatic brain injury. Hippocampus. 2014 Aug 1; PubMed.
- Small SA. Isolating pathogenic mechanisms embedded within the hippocampal circuit through regional vulnerability. Neuron. 2014 Oct 1;84(1):32-9. PubMed.
- Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci. 2011;12(10):585-601. PubMed.
Primary Papers
- Jackson J, Amilhon B, Goutagny R, Bott JB, Manseau F, Kortleven C, Bressler SL, Williams S. Reversal of theta rhythm flow through intact hippocampal circuits. Nat Neurosci. 2014 Aug 31; PubMed.
- Yang S, Yang S, Moreira T, Hoffman G, Carlson GC, Bender KJ, Alger BE, Tang CM. Interlamellar CA1 network in the hippocampus. Proc Natl Acad Sci U S A. 2014 Sep 2;111(35):12919-24. Epub 2014 Aug 19 PubMed.
- Thome C, Kelly T, Yanez A, Schultz C, Engelhardt M, Cambridge SB, Both M, Draguhn A, Beck H, Egorov AV. Axon-carrying dendrites convey privileged synaptic input in hippocampal neurons. Neuron. 2014 Sep 17;83(6):1418-30. Epub 2014 Sep 4 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.