Secreted APP Binds GABA-B Receptors, Blocks Neurotransmitter Release
Quick Links
Though Aβ tends to hog the research spotlight, scientists have steadily built a case for the importance of another APP fragment—secreted APP. Now, a paper in the January 11 Science explains how it tweaks synaptic transmission. Researchers led by Bart De Strooper and Joris de Wit at KU Leuven in Belgium report that sAPP acts as a GABAB receptor agonist. These receptors stifle the release of neurotransmitters, and sAPP binding promotes this inhibitory action. A 17 amino acid peptide from sAPP was sufficient to latch onto the extracellular side of the metabotropic receptor, effectively shutting down synaptic transmission in hippocampal circuits. The researchers presented most of the data at an EMBO symposium in June 2017 (Jul 2017 conference news).
- The secreted extracellular domain of APP binds GABAB-R1a.
- sAPP binding blocks the release of neurotransmitters.
- A 17mer peptide from sAPP shuts down synaptic transmission in mouse hippocampus.
The findings cast sAPP as an opposing synaptic force to Aβ, which instigates neuronal hyperactivity, commented Jorge Palop of the University of California, San Francisco. This raises the intriguing possibility that sAPP could be used therapeutically to reign in the excitatory effects of Aβ, he added (Sep 2007 news).
Before γ-secretase sinks its teeth into what’s left of the amyloid precursor protein (APP), α-, β-, or η-secretases first have their turn, releasing sAPPα, sAPPβ, or sAPPη from the membrane into the extracellular environs. Studies have reported that these shed fragments modulate synaptic transmission and neuroplasticity (Furukawa et al., 1996; Aug 2015 news). In fact, sAPPα is sufficient to rescue synaptic defects that crop up in APP knockout mice (research timeline on Ring et al., 2007; Young-Pearse et al., 2007; Hick et al., 2015). APP is also amply represented in the synapse (May 2014 news). However, exactly how sAPP meddles with synaptic transmission has been a mystery.
To solve it, first author Heather Rice and colleagues set out to find synaptic proteins that interact with sAPP. Mass spectroscopy analysis identified γ-aminobutyric acid type B receptor subunit 1 (GABAB-R1) as the primary protein interacting with sAPPα and sAPPβ. This meshed with findings from a recent study that placed APP within a GABAB-R1 interactome (Schwenk et al., 2016).
GABAB receptors consist of two subunits— GABAB-R1, which binds to GABA, and GABAB-R2, which couples to G proteins. These receptors are expressed on both pre- and postsynapses, where they trigger signaling cascades that inhibit neurotransmitter release, and dampen membrane excitability, respectively. They are a different animal than GABAA receptors, which function as fast-acting ion channels that squelch action potentials (Jan 2019 news). Two isoforms of the GABAB-R1 subunit exist, and the researchers found that sAPP only bound GABAB-R1a, which contains an extracellular sushi domain. Sushi domains are proposed to mediate axonal targeting of receptors (Biermann et al., 2010). In further experiments, the researchers found that an extension domain (ExD) of sAPP, previously described as a flexible linker connecting the rigid E1 and E2 domains of sAPP, bound to the sushi motif (Coburger et al., 2013; image below).
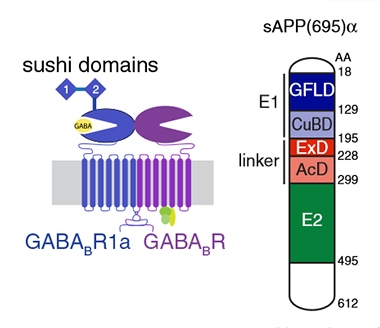
Pairs Well with Sushi. Sushi domains project from the extracellular side of GABAB-R1a subunits (left). sAPP binds sushi domains via its extension domain (ExD). [Courtesy of Rice et al., Science 2019.]
How would sAPP binding affect activity of the receptor? The researchers addressed this by investigating how sAPP affected the major known function of GABAB-R1: blocking neurotransmitter release from presynapses. Soluble APPα, sAPPβ, or ExD alone dramatically reduced the frequency, but not amplitude, of miniature excitatory postsynaptic currents (mEPSCs) on hippocampal neurons. However, sAPP also reduced miniature inhibitory postsynaptic currents (mIPSCs). Together, these findings suggested that sAPP binding blocked the release of excitatory neurotransmitters as well as the release of GABA from inhibitory synapses. To gauge which of these effects would win out in a functional circuit, the researchers added sAPP to hippocampal slices, where they found that as little as 1μM shut down synaptic transmission in Schaffer collateral synapses. The net effect of sAPP, therefore, was to douse synaptic transmission. Would this hold true within the complex circuitry of the human brain, or in AD? Researchers have reported that GABAergic function wanes in AD (Sep 2008 news; Dec 2018 news). Rice thinks sAPP likely affects both GABAergic and glutaminergic pathways.
Next, Rice narrowed down the segment of sAPP-ExD that bound to the GABAB-R1a. The 17 amino-acid peptide bound to the receptor with high affinity, on par with that of the ExD or sAPP. Nuclear magnetic resonance spectroscopy indicated that a smaller 9mer fit neatly into a binding pocket of the sushi domain, bestowing the disordered region with secondary structure.

Sushi Roll. The GABAB-R1a sushi domain forms a pocket surrounding the APP 9mer. [Courtesy of Rice et al., Science, 2019.]
Finally, Rice asked how the sAPP-derived 17mer would affect neuronal activity in vivo. The researchers infused the peptide directly into the hippocampi of anesthetized mice that expressed a fluorescent calcium indicator. Two-photon imaging indicated few calcium transients in CA1 pyramidal neurons, suggesting neurotransmission was shut down. A scrambled version of the peptide had no effect. Calcium bursts returned to normal after two hours. The findings raise the possibility that the 17mer peptide alone could be used as a therapeutic modulator of synaptic transmission.
How might all of this play out in the context of AD? This remains to be seen, Rice said, however, she pointed out that all fragments of sAPP, regardless of whether they were products of α-, β-, or η-secretases, acted as GABAB-R1a agonists. Therefore, changes in the total amount of sAPP would be necessary to affect the response, she said, potentially ruling out any major effect of blocking BACE, for example. While it is unclear whether the total sAPP changes in AD, levels of the fragment surely soar in animal models overexpressing APP, the researchers pointed out. This could explain some of the synaptic dysfunction that crops up in those models.
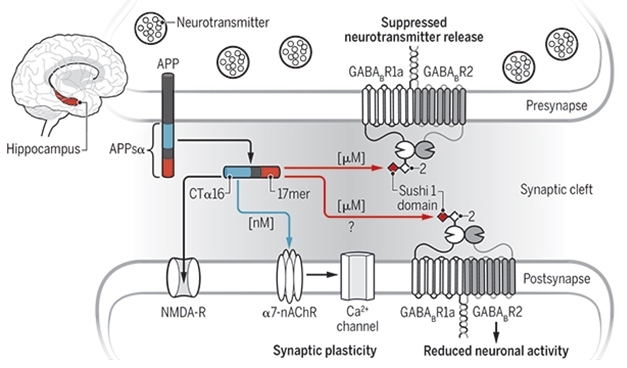
sAPP: Synaptic Socialite. A 17mer in the ExD of secreted APP binds to GABAB-R1 sushi domain (red), while other regions of the fragment (blue) associate with other neurotransmitter receptors. [Courtesy of Korte, Perspectives, Science, 2019.]
This is not sAPP’s first reported dalliance with neurotransmitter receptors. In an editorial accompanying the paper, Martin Korte of the Technical University of Braunschweig in Germany pointed out that the fragment also modulates N-methyl-d-aspartate and α-7 nicotinic receptors (Taylor et al., 2008; Moreno et al., 2015; Richter et al., 2018). Rice speculated that the type of receptor modulation might vary across the brain, depending on the local milieu of receptors, and variations in the expression of APP itself (Del Turco et al., 2016).
Despite sAPP’s interactions with other receptors, Tracy Young-Pearse of Brigham and Women’s Hospital in Boston thinks the unbiased approach Rice used strengthens the case for GABAB-R1.—Jessica Shugart
References
News Citations
- sAPP Binds GABA Receptor, and More News on APP
- Do "Silent" Seizures Cause Network Dysfunction in AD?
- Enter Aη: Alternative APP Cleavage Creates Synaptotoxic Peptide
- Synaptic Metropolis—Microscopic Census Documents 300,000 Protein Inhabitants
- GABA-A Receptor Structures Point to Drug Mechanisms
- Hyperactive Neurons and Amyloid, Side by Side
- Stem Cell Model Nails Link Between Tauopathy and GABAergic Dysfunction
Paper Citations
- Furukawa K, Barger SW, Blalock EM, Mattson MP. Activation of K+ channels and suppression of neuronal activity by secreted beta-amyloid-precursor protein. Nature. 1996 Jan 4;379(6560):74-8. PubMed.
- Ring S, Weyer SW, Kilian SB, Waldron E, Pietrzik CU, Filippov MA, Herms J, Buchholz C, Eckman CB, Korte M, Wolfer DP, Müller UC. The secreted beta-amyloid precursor protein ectodomain APPs alpha is sufficient to rescue the anatomical, behavioral, and electrophysiological abnormalities of APP-deficient mice. J Neurosci. 2007 Jul 18;27(29):7817-26. PubMed.
- Young-Pearse TL, Bai J, Chang R, Zheng JB, LoTurco JJ, Selkoe DJ. A critical function for beta-amyloid precursor protein in neuronal migration revealed by in utero RNA interference. J Neurosci. 2007 Dec 26;27(52):14459-69. PubMed.
- Hick M, Herrmann U, Weyer SW, Mallm JP, Tschäpe JA, Borgers M, Mercken M, Roth FC, Draguhn A, Slomianka L, Wolfer DP, Korte M, Müller UC. Acute function of secreted amyloid precursor protein fragment APPsα in synaptic plasticity. Acta Neuropathol. 2015 Jan;129(1):21-37. Epub 2014 Nov 29 PubMed.
- Schwenk J, Pérez-Garci E, Schneider A, Kollewe A, Gauthier-Kemper A, Fritzius T, Raveh A, Dinamarca MC, Hanuschkin A, Bildl W, Klingauf J, Gassmann M, Schulte U, Bettler B, Fakler B. Modular composition and dynamics of native GABAB receptors identified by high-resolution proteomics. Nat Neurosci. 2016 Feb;19(2):233-42. Epub 2015 Dec 21 PubMed.
- Biermann B, Ivankova-Susankova K, Bradaia A, Abdel Aziz S, Besseyrias V, Kapfhammer JP, Missler M, Gassmann M, Bettler B. The Sushi domains of GABAB receptors function as axonal targeting signals. J Neurosci. 2010 Jan 27;30(4):1385-94. PubMed.
- Coburger I, Dahms SO, Roeser D, Gührs KH, Hortschansky P, Than ME. Analysis of the overall structure of the multi-domain amyloid precursor protein (APP). PLoS One. 2013;8(12):e81926. Epub 2013 Dec 4 PubMed.
- Taylor CJ, Ireland DR, Ballagh I, Bourne K, Marechal NM, Turner PR, Bilkey DK, Tate WP, Abraham WC. Endogenous secreted amyloid precursor protein-alpha regulates hippocampal NMDA receptor function, long-term potentiation and spatial memory. Neurobiol Dis. 2008 Aug;31(2):250-60. Epub 2008 May 10 PubMed.
- Moreno L, Rose C, Mohanraj A, Allinquant B, Billard JM, Dutar P. sAβPPα Improves Hippocampal NMDA-Dependent Functional Alterations Linked to Healthy Aging. J Alzheimers Dis. 2015;48(4):927-35. PubMed.
- Richter MC, Ludewig S, Winschel A, Abel T, Bold C, Salzburger LR, Klein S, Han K, Weyer SW, Fritz AK, Laube B, Wolfer DP, Buchholz CJ, Korte M, Müller UC. Distinct in vivo roles of secreted APP ectodomain variants APPsα and APPsβ in regulation of spine density, synaptic plasticity, and cognition. EMBO J. 2018 Jun 1;37(11) Epub 2018 Apr 16 PubMed.
- Del Turco D, Paul MH, Schlaudraff J, Hick M, Endres K, Müller UC, Deller T. Region-Specific Differences in Amyloid Precursor Protein Expression in the Mouse Hippocampus. Front Mol Neurosci. 2016;9:134. Epub 2016 Nov 29 PubMed.
Other Citations
Further Reading
Papers
- Mockett BG, Richter M, Abraham WC, Müller UC. Therapeutic Potential of Secreted Amyloid Precursor Protein APPsα. Front Mol Neurosci. 2017;10:30. Epub 2017 Feb 7 PubMed.
- Müller UC, Deller T, Korte M. Not just amyloid: physiological functions of the amyloid precursor protein family. Nat Rev Neurosci. 2017 May;18(5):281-298. Epub 2017 Mar 31 PubMed.
- Taylor CJ, Ireland DR, Ballagh I, Bourne K, Marechal NM, Turner PR, Bilkey DK, Tate WP, Abraham WC. Endogenous secreted amyloid precursor protein-alpha regulates hippocampal NMDA receptor function, long-term potentiation and spatial memory. Neurobiol Dis. 2008 Aug;31(2):250-60. Epub 2008 May 10 PubMed.
Primary Papers
- Rice HC, de Malmazet D, Schreurs A, Frere S, Van Molle I, Volkov AN, Creemers E, Vertkin I, Nys J, Ranaivoson FM, Comoletti D, Savas JN, Remaut H, Balschun D, Wierda KD, Slutsky I, Farrow K, De Strooper B, de Wit J. Secreted amyloid-β precursor protein functions as a GABABR1a ligand to modulate synaptic transmission. Science. 2019 Jan 11;363(6423) PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of Hawaii
This is an intriguing paper reporting the novel finding that sAPPα, one of the secreted ectodomains of the amyloid precursor protein noted for its neurotrophic and neuroprotective actions, can act to regulate synaptic function via binding to a specific type B GABA receptor, GABAB-R1a, identified by a comprehensive proteomic screen. GABAB receptors are relatively slow, metabotropic, heteromeric neurotransmitter receptors and have significant presynaptic localization in brain, but are also present postsynaptically. GABAB receptors mediate much of the presynaptic inhibition driven by local GABA, the dominant inhibitory neurotransmitter in the brain. When present on excitatory inputs, excitation is suppressed, as shown in the present report for the 1a isoform, and when present on inhibitory GABAeric inputs as autoreceptors, the effect is disinhibition, promoting excitability. The finding that sAPPα regulates presynaptic inhibition and, in turn, presynaptic dynamics and synaptic transmission have ramifications for both physiological and perhaps pathological roles for APP beyond Aβ. Identification of a subdomain within sAPPα, specifically the extension domain (ExD), accounting for its high-affinity binding to and regulation of GABAB-R1a has implications for therapeutics. The reported findings raise a number of new questions, both for understanding the mechanisms at play and future application of the work.
These inhibitory metabotropic receptors are, of course, activated by GABA and couple differentially to adenylate cyclase, voltage-gated calcium channels and/or inward rectifying potassium channels, depending on locale and/or subtype. The question arises as to how sAPPα, and particularly ExD, regulates the activity and downstream intracellular coupling of GABAB-R1a through the latter’s sushi 1 domain, which is upstream of the GABA ligand binding domain. The effect of the GABAB-R antagonist might suggest an allosteric regulation of GABA binding. As for the regulation of presynaptic vesicle release, evident as changes in the frequency of miniature postsynaptic currents, might this be driven by negative regulation of presynaptic calcium channels or calcium levels? Perhaps a calcium imaging approach for presynaptic endings could be used (hippocampal cultures or isolated synaptosomes). In regard to synaptic plasticity, might the impact of sAPP differ for GABA-regulated pathways in different brain regions, possibly having the opposite action, namely an increase in excitability in certain pathways owing to GABAB-R1a serving as autoreceptors, for example? How might the regulation of sAPP release at these different pathways figure in? Moreover, changes in sAPP release with Alzheimer’s disease may translate into altered presynaptic GABAergic regulation via GABAB-R1a and hence altered neuronal excitability. Lastly, further refinement of the essential structural elements within the 34-amino acid ExD for GABAB-R1a regulation yielded a 9-amino acid sequence, though of lower affinity. This short peptide sequence could serve as a platform for eventually developing a unique small-molecule regulator of GABAB-R1a function in the brain.
Brigham and Women's Hospital and Harvard Medical School
APP has been shown to have a multifunctional role in both neurodevelopment and in the mature brain through its interactions with a variety of proteins. In this study, Rice et al. identify a novel role for APP in synaptic transmission through its interaction with a particular subunit of the GABA receptor. Importantly, the initial approach taken that identified the interaction was unbiased, and specifically searched for APP-interacting proteins present in the synaptic compartment. Through an elegant set of experiments, the authors identify a small region of the extracellular domain of APP that is sufficient to mediate the effects of the APP-GABAB-R1a interaction in vivo in the rodent brain.
Gladstone Institutes and University of California, San Francisco
This manuscript reinforces the notion that APP metabolites, including Aβ, participate in pre-and postsynaptic homeostatic mechanisms that regulate synaptic activity (for review, see Palop and Mucke, 2016) and identifies secreted APP as a key element regulating presynaptic release via GABAB receptors. Aβ reduces paired-pulse facilitation—a measure of increased release probability—and impairs long-term potentiation. Increased neurotransmitter release enhances the transfer of single-action potentials and synaptic depression, and may promote hyperactivity. Remarkably, Rice et al. now show that secreted APP may counteract theses effects by reducing release probability and enhancing paired-pulse facilitation. Although it is unclear whether secreted APP plays a role in the pathogenesis of AD, reducing presynaptic release to counteract AD-induced synaptic changes might be therapeutic. This manuscript also highlights the functional relevance of APP metabolites in synaptic function and the need to study APP and all its metabolites as a key molecule regulating synaptic function in health and disease.
References:
Palop JJ, Mucke L. Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat Rev Neurosci. 2016 Dec;17(12):777-792. Epub 2016 Nov 10 PubMed.
Baylor College of Medicine
Identification of sAPP receptor has been a long-standing interest for AD researchers, and a long list of potential receptors has been suggested over the past decade. In this elegant study, De Strooper, de Wit, and colleagues provide compelling evidence for GABAB as the cognate receptor for sAPP. The authors narrowed down the interaction of Sushi domains of GABAB to a 17 amino acid motif on the extension domain of sAPP, through which sAPP exerts a positive effect on GABAB receptor to reduce synaptic transmission. This finding, together with our earlier report showing that APP, highly expressed in GABAergic interneurons, critically mediates the balance between excitatory and inhibitory inputs through presynaptic GABAergic modulation (Wang et al., 2014), support a prominent role of APP in regulating GABAergic synaptic transmission. Since GABAB receptors mediate both pre- and postsynaptic inhibition, and may function as autoreceptors in GABAergic neurons or heteroreceptors in glutamate neurons, it would be interesting to further dissect these cellular mechanisms, and more importantly, understand how these mechanisms operate in the context of AD.
References:
Wang B, Wang Z, Sun L, Yang L, Li H, Cole AL, Rodriguez-Rivera J, Lu HC, Zheng H. The amyloid precursor protein controls adult hippocampal neurogenesis through GABAergic interneurons. J Neurosci. 2014 Oct 1;34(40):13314-25. PubMed.
Make a Comment
To make a comment you must login or register.