TDP-43 Snarls Nuclear Traffic
Quick Links
Many proteins linked to neurodegeneration impede traffic in and out of the nucleus, and in the January 8 Nature Neuroscience, researchers add TAR DNA binding protein–43 to the mix. This predominantly nuclear protein forms cytoplasmic inclusions in neurons and glia of nearly all patients with amyotrophic lateral sclerosis, and many with frontotemporal dementia. Researchers led by Wilfried Rossoll at the Mayo Clinic, Jacksonville, Florida, used a novel protein-labeling technique to probe the contents of those aggregates. They identified dozens of components of the nucleocytoplasmic transport machinery. The researchers went on to uncover how TDP-43 interferes with the transport of protein into and RNA out of mouse and human nuclei. In addition, they spotted damaged nuclear pores in brain tissues from people who had had sporadic or familial ALS.
- TDP-43 aggregates include components of the nucleocytoplasmic transport machinery.
- TDP-43 pathology disrupts nuclear protein import and RNA export in mouse and human cells.
- Nuclear pores are disrupted in brain samples from patients with sporadic or familial ALS.
“These important findings support the idea that impairment of nucleocytoplasmic transport is a common element of the cytopathology of several neurodegenerative diseases,” wrote Ulrich Hartl, Max-Planck Institute for Biochemistry, Martinsried, Germany, in an email to Alzforum. Mark Hipp, also at the Max-Planck Institute, agreed, noting, “It’s exciting to see an expert lab like Rossoll’s examine this question so thoroughly in different cells, in Drosophila, and in patients. What I think is most exciting is that, regardless of where we start, we seem to hit the same [nucleocytoplasmic transport] target over and over again.” Indeed, disruptions of nucleocytoplasmic transport repeatedly surfaced last year in studies of ALS/FTD, as well as Huntington’s and Alzheimer’s disease (Kim and Taylor, 2017; Dec 2017 conference news).
Clues that nucleocytoplasmic transport might be disrupted in ALS date back to 1995 (Vuopala et al., 1995). However, the first clear mechanistic link only emerged in 2015 when researchers discovered that pathologic hexanucleotide repeats in the C9ORF72 gene, which cause ALS and FTD, snarl traffic through pores in the nuclear envelope (Aug 2015 news). Subsequently, Hipp and Hartl found mRNA accumulating in the nuclei of human embryonic kidney cells overexpressing a C-terminal fragment of TDP-43 that forms ALS-like inclusions. They also uncovered nuclear transport factors littering the cytoplasm of these cells (Woerner et al., 2016).
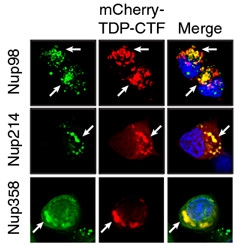
Nuclear Meltdown.
TDP-43 aggregates (red) contain nuclear pore complex proteins (green) Nup98, Nup214, and Nup358. [Courtesy of Chou et al., Nature Neuroscience.]
Rossoll’s discovery connects the dots. Then at Emory University, Atlanta, Rossoll, first author Ching-Chieh Chou, and colleagues dissected the TDP-43 inclusions using BioID, a proteomics technique that allows researchers to identify proteins that cozy up to each other in mammalian cells (Roux et al., 2012). BioID relies on a ligase that attaches biotin only to proteins in its immediate proximity. In N2a neuroblastoma cells, Chou expressed BioID constructs containing full-length TDP-43 or a C-terminal fragment (TDP-CTF) that forms inclusions (Igaz et al., 2009). He then fished out any labeled proteins with biotin-binding neuravidin beads. The approach combines harsh conditions to dissolve the aggregates, without losing track of the associated proteins.
The researchers used gene-ontology analysis to compare TDP-43 and TDP-CTF interactors. Consistent with TDP-43’s role as a regulator of mRNA splicing, trafficking, and stabilization, the BioID construct pulled out mRNA processing proteins. The largest group of TDP-CTF interactors fell into the category of intracellular protein transport, with nucleocytoplasmic transport components forming a major subset. “We found many proteins, but we first focused on the nucleocytoplasmic transport machinery because they really stood out in the proteomics analysis, and also because of the parallels with previous C9 results. We hadn’t anticipated seeing these components, but in hindsight it makes a lot of sense,” said Rossoll.
To validate, the researchers co-expressed GFP-tagged chimeras of each of 37 interactor proteins with mCherry-tagged TDP-CTF. The interactors included proteins of the nuclear pore complex, aka nucleoporins, scaffold nucleoporins, and nuclear export factors. In N2a cells, most co-aggregated with the truncated TDP-43 (see graphic below). Two transmembrane nucleoporins and two nuclear lamina proteins were displaced from the nuclear pore, suggesting a structural disruption of this complex. Interestingly, expression of nucleoporins Nup85 and Tpr, and the nuclear import protein karyopherin β1 (Kpnb1), reduced TDP-CTF aggregates. Others have reported that Kpnb1 acts as a dis-aggregase, not only preventing aggregates of TDP-43, but dissolving aggregates once they have formed (May 2017 conference news).
Rossoll wondered whether low-complexity domains (LCDs), known to drive self-assembly and protein-protein interactions, might mediate the co-aggregation of TDP-CTF with nuclear pore proteins. TDP-43 harbors an LCD in its C-terminus and some nucleoporins also have them. Combing through human nucleoporin sequences, the researchers found a particular type of LCD, a prion-like domain, in five so-called FG Nups. These nucleoporins sport filamentous domains studded with phenylalanine-glycine (FG) repeats that reach into the nuclear pore to regulate traffic. LCD fragments of three of these proteins, Nup98, Nup153, and Nup214, promoted aggregation with TDP-CTF. Nup54 and Nup358 also contain such domains. LCDs help FG Nups form a sieve-like hydrogel that regulates the permeability of nuclear pores, noted Rossoll, “but this probably makes them more vulnerable to aggregation.”
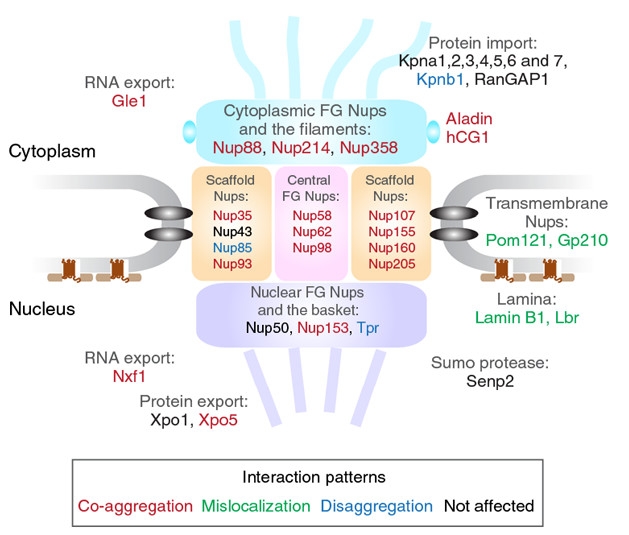
Nucleus-TDP-43 Link. This representation of a nuclear pore summarizes how TDP-43 aggregates interact with components of the nucleocytoplasmic transport machinery, including FG Nups that contain phenylalanine-glycine (FG) repeats. [Courtesy of Chou et al., Nature Neuroscience.]
Beyond protein interactions, electron microscopy revealed misshapen nuclei, with occasional invaginations of the nuclear membrane, in N2a cells expressing TDP-CTF. Under the light microscope, Nup98 clustered oddly in the nuclear membrane. In cortical primary neurons expressing TDP-CTF or TDP-43 carrying an ALS-linked mutation, TDP-43Q331K or TDP-43M337V, the nuclear lamina was distorted in up to 40 percent of nuclei. Phosphorylated gH2AX, a histone marker of double-strand breaks, indicated DNA damage. The cells accumulated a nuclear transport reporter in their cytoplasm while retaining poly(A) RNA in the nucleus, proving the disruptions affect nuclear transport.
Fibroblasts isolated from patients with sporadic ALS, TDP-ALS, or ALS caused by C9PORF72 repeat expansion (C9-ALS) had similarly disrupted nuclei, as did TDP-ALS motor neurons derived from induced pluripotent stems cells. Finally, the authors found that another nucleoporin, Nup205, was abnormally distributed in brain tissues from one TDP-43, seven C9, and 10 sporadic ALS cases. In the motor cortex, Nup205 antibodies lit up inclusions in the cytoplasm of TDP-ALS and sALS cells, while binding nuclei only weakly. In C9-ALS, Nup205 was mostly found unevenly dotting the nuclear periphery. Samples from one other patient lacked Nup205 pathology, but that person also had no TDP-43 aggregates. Antibodies to other nucleoporins were unsuitable for immunohistochemistry of human tissue preparations, said Rossoll. Nevertheless, the findings suggest nuclear pore abnormalities accompany TDP-43 pathology in disease, even in the absence of C9ORF72 repeat expansion.

Pore Pathology.
Nucleoporin Nup205 (brown) co-localizes (arrows) with TDP-43 (blue) in cytoplasmic aggregates in the motor cortex (left) and hippocampus (right) of a patient with TDP-ALS. [Courtesy of Chou et al., Nature Neuroscience.]
Rossoll proposes a positive feed-forward loop, in which C9ORF72 expansions cause nuclear pore obstruction that, in turn, causes TDP-43 to accumulate in the cytoplasm, where it causes more nuclear pore damage and snags transport further. Other factors, including aging, mutations, and/or environmental triggers, can have the same effect of shifting TDP-43 into the cytoplasm. “TDP-43 is not only a victim, but a perpetrator,” said Rossoll.
Fen-Biao Gao, University of Massachusetts, Worcester, agreed. “Since TDP-43 pathology is seen in 95 to 97 percent of ALS cases, this study is important, because it shows that disruption of nucleocytoplasmic transport is much more widespread in ALS than we previously thought.”
What about therapeutic implications? Rossoll thinks inhibiting nuclear protein export might be protective, as observed in models of C9-ALS, tauopathy, and Huntington’s disease (Grima et al., 2017). Indeed, KPT-335 and KPT-276, inhibitors of nuclear export, rescued primary mouse cortical neurons transfected with TDP-CTF or TDP-43Q331K and reduced the number of cells with abnormal nuclear shapes.
Developed by Karyopharm Therapeutics in Newton, Massachusetts, KPT-335 passed safety hurdles and appeared well-tolerated in a Phase 1 trial done in 2015. Using a dose-escalation scheme, cohorts of eight participants were given two doses of KPT-335 or placebo two days apart. Doses ranged from 5–100 mg. With a $900,000 award from the Target ALS Foundation, Karyopharm plans to evaluate a related compound, KPT-350, for treatment of ALS.—Marina Chicurel
References
News Citations
- Is There No End to Tau’s Toxic Tricks?
- ALS Gene Repeats Obstruct Traffic Between Nucleus and Cytoplasm
- Protein Liquid-Liquid Phase Transitions: The Science Is About to Gel
Paper Citations
- Kim HJ, Taylor JP. Lost in Transportation: Nucleocytoplasmic Transport Defects in ALS and Other Neurodegenerative Diseases. Neuron. 2017 Oct 11;96(2):285-297. PubMed.
- Vuopala K, Ignatius J, Herva R. Lethal arthrogryposis with anterior horn cell disease. Hum Pathol. 1995 Jan;26(1):12-9. PubMed.
- Woerner AC, Frottin F, Hornburg D, Feng LR, Meissner F, Patra M, Tatzelt J, Mann M, Winklhofer KF, Hartl FU, Hipp MS. Cytoplasmic protein aggregates interfere with nucleocytoplasmic transport of protein and RNA. Science. 2016 Jan 8;351(6269):173-6. Epub 2015 Dec 3 PubMed.
- Roux KJ, Kim DI, Raida M, Burke B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J Cell Biol. 2012 Mar 19;196(6):801-10. Epub 2012 Mar 12 PubMed.
- Igaz LM, Kwong LK, Chen-Plotkin A, Winton MJ, Unger TL, Xu Y, Neumann M, Trojanowski JQ, Lee VM. Expression of TDP-43 C-terminal Fragments in Vitro Recapitulates Pathological Features of TDP-43 Proteinopathies. J Biol Chem. 2009 Mar 27;284(13):8516-24. Epub 2009 Jan 21 PubMed.
- Grima JC, Daigle JG, Arbez N, Cunningham KC, Zhang K, Ochaba J, Geater C, Morozko E, Stocksdale J, Glatzer JC, Pham JT, Ahmed I, Peng Q, Wadhwa H, Pletnikova O, Troncoso JC, Duan W, Snyder SH, Ranum LP, Thompson LM, Lloyd TE, Ross CA, Rothstein JD. Mutant Huntingtin Disrupts the Nuclear Pore Complex. Neuron. 2017 Apr 5;94(1):93-107.e6. PubMed.
External Citations
Further Reading
Papers
- Fox BW, Tibbetts RS. Neurodegeneration: Problems at the nuclear pore. Nature. 2015 Sep 3;525(7567):36-7. Epub 2015 Aug 26 PubMed.
- Shi KY, Mori E, Nizami ZF, Lin Y, Kato M, Xiang S, Wu LC, Ding M, Yu Y, Gall JG, McKnight SL. Toxic PRn poly-dipeptides encoded by the C9orf72 repeat expansion block nuclear import and export. Proc Natl Acad Sci U S A. 2017 Feb 14;114(7):E1111-E1117. Epub 2017 Jan 9 PubMed.
- Frost B, Bardai FH, Feany MB. Lamin Dysfunction Mediates Neurodegeneration in Tauopathies. Curr Biol. 2016 Jan 11;26(1):129-36. Epub 2015 Dec 24 PubMed.
Primary Papers
- Chou CC, Zhang Y, Umoh ME, Vaughan SW, Lorenzini I, Liu F, Sayegh M, Donlin-Asp PG, Chen YH, Duong DM, Seyfried NT, Powers MA, Kukar T, Hales CM, Gearing M, Cairns NJ, Boylan KB, Dickson DW, Rademakers R, Zhang YJ, Petrucelli L, Sattler R, Zarnescu DC, Glass JD, Rossoll W. TDP-43 pathology disrupts nuclear pore complexes and nucleocytoplasmic transport in ALS/FTD. Nat Neurosci. 2018 Feb;21(2):228-239. Epub 2018 Jan 8 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Barshop Institute for Longevity and Aging Studies/University of Texas Health Science Center
This paper from the Rossoll group is an important contribution to the emerging concept that disruption of nuclear architecture and nucleocytoplasmic transport are central mediators of neurodegeneration in multiple disorders. The nature of the nuclear envelope changes may differ somewhat among neurodegenerative disorders. We see that nuclear envelope aberrations in AD patient brain are quite clearly invaginations, whereas the nuclear envelope in ALS, FTD, and Huntington's disease patient material are typically described as "irregular," "ruffled," or "distorted." It is also important to determine if neurodegeneration causes general disruption of the nuclear envelope, versus active remodeling of the nuclear envelope in response to a stimulus. We've published evidence for the latter in the context of tauopathy.
The overlap with work in the cancer field is interesting.
Make a Comment
To make a comment you must login or register.