Like a Tiny Spider-Man, Aβ May Fight Infection by Cocooning Microbes
Quick Links
For all that researchers know about Aβ, the peptide so strongly linked to Alzheimer’s disease, they have scant knowledge of its normal function in or out of the cell. A new paper strengthens the idea that Aβ helps fights infection as part of the innate immune system. In the May 25 Science Translational Medicine, scientists led by Robert Moir and Rudolph Tanzi of Massachusetts General Hospital in Charlestown, Massachusetts, report that Aβ helps human cells, worms, and mice ward off invasions by pathogenic yeast and bacteria. The sticky peptide oligomerizes, binds to the surface of these microbes, then fibrillizes to ensnare them in sticky tendrils until other defense mechanisms move in for the kill. Featured in The New York Times, the findings do not directly address the hypothesis that microbes might precipitate AD; rather, they assign Aβ a physiological role. However, they do hint that microbes might seed plaques, which could indirectly explain why different pathogens have been tied to AD.
“This is a superb piece of work,” wrote Guillaume van Niel, Institut Curie, Paris, to Alzforum. “One of the most famous pathological amyloids may finally belong to the emerging class of functional amyloids.” He added that this paper challenges the notion that amyloidogenesis results from misprocessing or mistrafficking, and instead hints that it may be tightly regulated and induced by microbial infection.
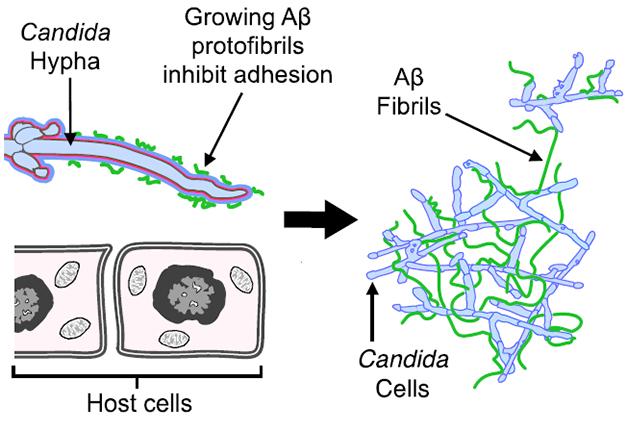
A Sticky Web. Aβ oligomers (green, left) bind to yeast to prevent them from attaching to host cells. Those oligomers elongate into fibrils (right) and trap the yeast in sticky clumps. [Courtesy of Science Translational Medicine/AAAS.]
Moir and Tanzi previously reported that synthetic Aβ inhibited the growth of eight pathogens in culture, including the yeast Candida albicans, and the bacteria Escherichia coli and Staphylococcus aureus (Soscia et al., 2010). It performed as well as or better than LL-37, a human antimicrobial peptide (AMP). AMPs are 15 to 20 amino acid peptides that act in a variety of ways throughout the body to combat fungi, bacteria, and viruses as part of the innate immune system. LL-37 typifies AMPs that oligomerize and bind to the surface of microbes, preventing their attachment to host cells, then fibrillizing around them until immune cells kill them off. The authors set out to determine if Aβ oligomerization and fibrillization—usually thought of as pathological—actually served functional roles akin to LL-37.
Co-first authors Deepak Kumar, Se Hoon Choi, and Kevin Washicosky first tested whether Aβ helped three different organisms fend off pathogens. Alzforum covered these results when they were presented at the Zilkha conference last month (May 2016 conference news). In a nutshell, overexpressing Aβ doubled survival of human brain neuroglioma (H4) cells and C. elegans in the face of a Candida infection. Likewise, 5xFAD mice, which overexpress Aβ, survived infection with Salmonella for up to 96 hours, compared to 60-72 hours for wild-type and 54 hours for APP knockouts.
Does Aβ work like LL-37? The scientists compared control H4 cells to H4 cells overexpressing Aβ42 or Aβ40. The Aβ cells bound fewer yeast cells, suggesting that the peptide somehow interferes with yeast attachment, which is required for infection.
Kumar and colleagues next looked to see if Aβ binds the microbes. LL-37 attaches to microbial surface sugars by way of a heparin-binding motif. This six-amino-acid sequence alternates hydrophobic with positively charged amino acids and remains hidden until LL-37 oligomerizes. Aβ also sports a heparin-binding domain between residues 12-17. A binding immunoassay revealed that Aβ bound to Candida cell walls, but only in oligomeric form. However, it failed to bind if incubated with soluble mannan and glucan, two sugars yeast release to gum up the heparin-binding domain on AMPs.
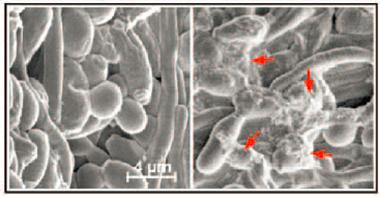
Snagged.
Scanning electron microscopy revealed fibrous material (red arrows) around yeast incubated with H4 cells that produce Aβ (right). [Courtesy of Science Translational Medicine/AAAS.]
After binding to a microbe’s surface, AMPs such as LL-37 fibrillize to form meshes that ensnare microbes. Can Aβ fibrillization do that, too? The researchers incubated yeast with control H4 cells or cells that overexpressed Aβ. Scanning electron microscope images of the culture medium revealed fibrous material around the yeast in the H4-Aβ42 culture (see image at right). Transmission electron microscopy revealed Aβ-fibrils stuck to the yeast surface.
The data suggest that in cell culture, overexpressing Aβ helps cells resist microbes via a mechanism similar to that of LL-37. To test whether Aβ works the same way in animals, the researchers infected Aβ-overexpressing C. elegans with yeast. In just two hours, the surface of yeast in the worm’s gut tested positive for Aβ by immunogold labelling. Later, clumps of yeast stained positive for thioflavin S, implying Aβ had formed plaques around them. In four-week-old 5xFAD mice, the authors saw something similar. At this age, these transgenic mice typically have no plaques but, two days after infection with Salmonella, Aβ had deposited throughout their brain. The bacteria and plaques were in the same place; indeed transmission electron microscopy revealed the microbes embedded inside plaques (see video below).
Plaques Trap Bacteria. Confocal fluorescence microscopy shows Salmonella (green) sheathed in b-amyloid (red) in 5XFAD mouse brain. [Courtesy of Science Translational Medicine/AAAS.]
What happens to the microbes inside these plaques? Moir said it appears these extracellular traps generate hydrogen peroxide, which both kills the microbe and cross-links the Aβ fibrils, making them resistant to proteases. That’s good for sealing in the bug, but makes plaques hard to clear, he said.
The data support the idea that Aβ functions as an AMP, and counter the prevailing view that it is intrinsically pathological, Moir said. “Its activities start to make more sense when you view Aβ as an AMP,” he told Alzforum, “It’s provocative to see this peptide so quickly form amyloids that are protective.” Moir noted that Aβ has been conserved in vertebrates for 400 million years, suggesting it has a function.
He believes his findings explain why many microbes have been tied to Alzheimer’s disease. “This is a general response,” said Moir. “Aβ will do the same thing with whatever you throw at it.” Of course, Aβ itself is known to be toxic to cells, especially neurons, suppressing synaptic activity and causing cell death. It could be that this innate immune process becomes dysregulated in Alzheimer’s disease and becomes pathological, said Moir.
Moir emphasized that the study does not claim infection causes Alzheimer’s disease. It only assigns Aβ a function. However, the microbial hypothesis deserves to be looked at more seriously, he said. He plans to systematically characterize microbes lurking in the brains of AD patients, and examine other amyloidogenic proteins implicated in disease—such as amylin in diabetes—to test whether they function as AMPs.
“Moir and colleagues did a marvelous job using these different models to demonstrate that Aβ oligomers and fibrils protect against infection,” said Brian Balin, Philadelphia College of Osteopathic Medicine. While this mechanism may help neutralize invaders that remain outside of cells, pathogens that enter cells—such as viruses—may trigger β-amyloid production without being killed, hiding safe inside their hosts, he noted. They may persist in the brain and keep generating an amyloid response, Balin said. This could explain the links between intracellular pathogens such as herpes and chlamydia and AD, which have been proposed by Balin and others, (Wozniak et al., 2009; Balin et al., 2008).
“It’s a provocative hypothesis that addresses the long-standing question of why Aβ aggregates,” said Douglas Fowler, University of Washington, Seattle, who called the idea that these aggregates could be functional compelling and interesting. “If it indeed turns out to be true, then they will have resolved one of the fundamental mysteries of how Aβ aggregation relates to its native function in the brain and possibly to Alzheimer’s disease,” he said.
Other scientists remain skeptical. For example, Bruce Kagan at the University of California, Los Angles, cautioned that this mechanism has still only been examined in animal models. Few of many known amyloid proteins have a known antimicrobial function, Kagan noted, though he and colleagues reported that the amyloid-forming serum amyloid A has potent antimicrobial activity (Hirakura et al., 2002). For his part, Kagan believes that Aβ’s destructive effect on pathogens comes from amyloid proteins damaging membranes and killing cells due to their β-sheet structure. —Gwyneth Dickey Zakaib
References
News Citations
Research Models Citations
Paper Citations
- Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson M, Hyman B, Burton MA, Goldstein LE, Duong S, Tanzi RE, Moir RD. The Alzheimer's disease-associated amyloid beta-protein is an antimicrobial peptide. PLoS One. 2010 Mar 3;5(3):e9505. PubMed.
- Wozniak MA, Mee AP, Itzhaki RF. Herpes simplex virus type 1 DNA is located within Alzheimer's disease amyloid plaques. J Pathol. 2009 Jan;217(1):131-8. PubMed.
- Balin BJ, Little CS, Hammond CJ, Appelt DM, Whittum-Hudson JA, Gérard HC, Hudson AP. Chlamydophila pneumoniae and the etiology of late-onset Alzheimer's disease. J Alzheimers Dis. 2008 May;13(4):371-80. PubMed.
- Hirakura Y, Carreras I, Sipe JD, Kagan BL. Channel formation by serum amyloid A: a potential mechanism for amyloid pathogenesis and host defense. Amyloid. 2002 Mar;9(1):13-23. PubMed.
External Citations
Further Reading
Papers
- Schluesener HJ, Su Y, Ebrahimi A, Pouladsaz D. Antimicrobial peptides in the brain: neuropeptides and amyloid. Front Biosci (Schol Ed). 2012;4:1375-80. PubMed.
- Bergman P, Roan NR, Römling U, Bevins CL, Münch J. Amyloid formation: functional friend or fearful foe?. J Intern Med. 2016 Aug;280(2):139-52. Epub 2016 May 6 PubMed.
- Chu H, Pazgier M, Jung G, Nuccio SP, Castillo PA, de Jong MF, Winter MG, Winter SE, Wehkamp J, Shen B, Salzman NH, Underwood MA, Tsolis RM, Young GM, Lu W, Lehrer RI, Bäumler AJ, Bevins CL. Human α-defensin 6 promotes mucosal innate immunity through self-assembled peptide nanonets. Science. 2012 Jul 27;337(6093):477-81. Epub 2012 Jun 21 PubMed.
- Harris SA, Harris EA. Herpes Simplex Virus Type 1 and Other Pathogens are Key Causative Factors in Sporadic Alzheimer's Disease. J Alzheimers Dis. 2015;48(2):319-53. PubMed.
- Miklossy J. Emerging roles of pathogens in Alzheimer disease. Expert Rev Mol Med. 2011;13:e30. PubMed.
Primary Papers
- Kumar DK, Choi SH, Washicosky KJ, Eimer WA, Tucker S, Ghofrani J, Lefkowitz A, McColl G, Goldstein LE, Tanzi RE, Moir RD. Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer's disease. Sci Transl Med. 2016 May 25;8(340):340ra72. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Philadelphia College of Osteopathic Medicine
Philadelphia College of Osteopathic Medicine
As a further comment to this article I want to be perfectly clear that this study helps support the work that many of us in the infection arena of Alzheimer’s research have been looking at for almost three decades. I cannot even begin to address the enormity of this work by so many investigators around the globe, although if one searches in PubMed, most of these articles are readily obtainable. However, there are a few points that I would like to address. Specifically, with regard to looking in human AD brain tissues which is now proposed as a follow-up study to the current Kumar et al. report, we reported on finding Chlamydia pneumoniae in AD brains in 1998, Ruth Itzhaki reported on HSV1 in human brains in 1991, and Judith Miklossy reported on Borrelia species in AD brains in 1993. These are just three reports on infectious agents in human AD brains out of many, many more in the literature. Furthermore, from my lab we have demonstrated that Chlamydia pneumoniae, a respiratory intracellular organism, inoculated through a normal route (i.e., intranasally) into normal non-transgenic mice actually resulted in amyloid plaques in the brain (Little et al., 2004, 2014). These studies already demonstrated that amyloid could be a consequence of infection entering the CNS and we believe this will result in pathological accumulations, especially in human brains. Itzhaki and Miklossy and others have demonstrated a relationship between infection and amyloid as well. These comments are not to detract from the current report, but they are needed to provide an appropriate context in which to evaluate the current findings.
References:
Jamieson GA, Maitland NJ, Wilcock GK, Craske J, Itzhaki RF. Latent herpes simplex virus type 1 in normal and Alzheimer's disease brains. J Med Virol. 1991 Apr;33(4):224-7. PubMed.
Miklossy J. Alzheimer's disease--a spirochetosis?. Neuroreport. 1993 Jul;4(7):841-8. PubMed.
Balin BJ, Gérard HC, Arking EJ, Appelt DM, Branigan PJ, Abrams JT, Whittum-Hudson JA, Hudson AP. Identification and localization of Chlamydia pneumoniae in the Alzheimer's brain. Med Microbiol Immunol. 1998 Jun;187(1):23-42. PubMed.
Little CS, Joyce TA, Hammond CJ, Matta H, Cahn D, Appelt DM, Balin BJ. Detection of bacterial antigens and Alzheimer's disease-like pathology in the central nervous system of BALB/c mice following intranasal infection with a laboratory isolate of Chlamydia pneumoniae. Front Aging Neurosci. 2014;6:304. Epub 2014 Dec 5 PubMed.
Little CS, Hammond CJ, MacIntyre A, Balin BJ, Appelt DM. Chlamydia pneumoniae induces Alzheimer-like amyloid plaques in brains of BALB/c mice. Neurobiol Aging. 2004 Apr;25(4):419-29. PubMed.
Make a Comment
To make a comment you must login or register.