TMEM175 Maintains Lysosome pH by Pushing Out Protons
Quick Links
Lysosomes require tight regulation of their pH to degrade their contents. Researchers know that V-ATPase pumps protons into these little digestive sacs, but what protein shuttles the ions back out? Researchers led by Haoxing Xu and Meiqin Hu, University of Michigan, Ann Arbor, now point to TMEM175, the potassium channel already known as a risk gene for Parkinson’s disease and Lewy body dementia (Mar 2021 news). In the June 23 Cell, they reported that TMEM175 shunts potassium out of lysosomes when they are neutral, but switches to protons when they are too “sour.” TMEM175 knockout cells have hyperacidified lysosomes—and hypoactive hydrolases.
- TMEM175 was previously pegged as a lysosomal potassium channel.
- Cells lacking it have overly acidic lysosomes, hobbling hydrolases.
- TMEM175 knockout mice accumulated α-synuclein.
“Lysosomes and proteases need some acid to be active, but this paper finds that overshooting acidity causes their loss of activity, too,” Matthew LaVoie, University of Florida, Gainesville, told Alzforum.
“[The suggestion] that TMEM175 is a lysosomal proton channel is quite striking,” Mark Cookson of the NIH wrote (full comment below). Dejian Ren, University of Pennsylvania, Philadelphia, agreed. “These researchers found a new pathway for how TMEM175 conducts ions and influences lysosome function,” he told Alzforum.
Just last March, Tianmin Fu, Ohio State University, Columbus, and colleagues had also reported that TMEM175 shifts from potassium to protons as lysosomes acidify. These authors used electron microscopy to show that, as the pH dropped from 7.4 to 5.5, amino acids within the channel’s pore swiveled inward, shrinking its corridor to keep out the large potassium ions while still allowing small protons to pass.
“The major discovery of Hu et al. and our paper is that TMEM175 is a proton channel in lysosomes, [answering] a longstanding and exceptionally important question in the field of lysosomal biology,” Fu wrote (full comment below).
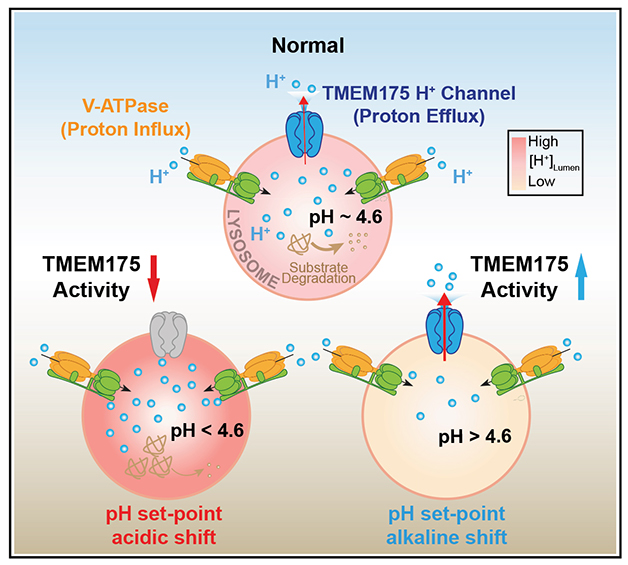
Just Right. To maintain the optimal lysosomal pH of 4.6 (top circle), V-ATPase pumps in protons while TMEM175 shuttles them back out. Lysosomes become too acidic when TMEM175 stops letting protons through (bottom left) or too alkaline if the channel is too leaky (bottom right). Both pH perturbations prevent protein degradation (tan squiggles). [Courtesy of Hu et al., Cell, 2022.]
In search of the lysosome proton efflux channel, first author Hu overexpressed 29 channel proteins in monkey fibroblasts, which contain lots of lysosomes. He isolated those vesicles and measured the current produced as ions flow through their membrane channels using a patch-clamp technique. Only lysosomes overexpressing TMEM175 evoked a current. As the scientists lowered the lysosomal pH from 7.2 to 3.5, the current got stronger (see image below). This indicates that protons flowed out of TMEM175 to counter hyperacidification as the pH dropped below the optimal 4.6.
TMEM175 is gated by protons within the lysosome, only shunting those ions once the pH becomes acidic. Intriguingly, in a pH-independent activation, the polyunsaturated fat arachidonic acid also stirred the channel to open, allowing protons out at pH 6.

Acid Exit. In a lysosome (circle) expressing TMEM175 (yellow dot), protons pass through the channel and out of the lysosome when it was acidic (pHL) and its surrounding solution (pHC) was neutral (left). The more acidic the lysosome, the stronger the current created by proton flow (right). [Courtesy of Hu et al., Cell, 2022.]
The same pH-dependent proton current occurred in HEK293T cells overexpressing TMEM175 but not in TMEM175 knockout HEK293Ts. TMEM175 re-expression restored ion flow.
As for potassium, the current produced by these ions in TMEM175-overexpressing cells halved as the pH dropped from 7.2 to 6. At pH 4.6, fewer than one in 10 of the ions rushing through TMEM175 were potassium—all others were protons. Therefore, potassium ion flux dwindled at the physiological pH of lysosomes while protons flowed abundantly.
This proton exodus through TMEM175 is crucial for lysosomes to achieve pH homeostasis. The vesicles were overly acidic in the knockout cells and returned to normal LysoTracker Red fluorescence after adding wild-type TMEM175 but not a TMEM175 mutant, D41A, that is impermeable to protons.
Does TMEM175 influence how lysosomes function, too? Indeed, in TMEM175 knockout cells, the lysosomal hydrolase cathepsin B cleaved less fluorogenic substrate. Expression of wild-type TMEM175, but not the D41A mutant, restored cathepsin B’s activity, hinting that too much lysosome acidification harms hydrolase activity.
In a test of whether TMEM175’s effects on lysosomes hold in vivo, hippocampal neurons from TMEM175 knockout mice produced no current in an acidic solution, had overly acidic lysosomes at around pH 4, and had half the cathepsin B activity as wild-type neurons.
Importantly, cathepsins break down α-synuclein, and TMEM175 helps lysosomes clear synuclein aggregates (Mar 2009 news; McGlinchey and Lee, 2015; Jinn et al., 2017). The scientists tested the α-synuclein-clearing ability of TMEM175 knockout mice by injecting the striata of 3-month-olds with preformed synuclein fibrils. One month later, the knockouts had more toxic, phosphorylated α-synuclein than wild-types, indicating that, without TMEM175, α-synuclein aggregates lingered.
“In light of this paper, it is now possible, and necessary, to revisit previous studies, relating known risk or protective variants of TMEM175 to lysosomal function and disease mechanisms,” wrote Thomas Gasser and Johannes Gloeckner, University of Tübingen, Germany. Dimitri Krainc and Nathaniel Safren, Northwestern University, Chicago, also noted broader implications. “The notion that such a ubiquitously expressed protein with such an overarching impact on lysosomal function should specifically be implicated in PD highlights the importance of lysosomal function to dopaminergic neuron viability," they wrote (comments below).
In fact, variants in genes for other lysosomal enzymes, such as CTSB encoding cathepsin B, ATP6V0A1 for V-ATPase subunit A1, and GBA1 for glucocerebrosidase, increase PD risk (Sep 2017 news). “Now that we have just a little more mechanistic insight into TMEM175, we can explore how other PD risk genes may intersect in this lysosomal pathway,” LaVoie said.—Chelsea Weidman Burke
References
News Citations
- Lewy Body Dementia Shares Risk Genes with Alzheimer’s, Parkinson’s
- Striking at Synuclein by Driving Degradation
- Lysosomes Take Center Stage in Parkinson’s and Frontotemporal Dementia
Alzpedia Citations
Paper Citations
- McGlinchey RP, Lee JC. Cysteine cathepsins are essential in lysosomal degradation of α-synuclein. Proc Natl Acad Sci U S A. 2015 Jul 28;112(30):9322-7. Epub 2015 Jul 13 PubMed.
- Jinn S, Drolet RE, Cramer PE, Wong AH, Toolan DM, Gretzula CA, Voleti B, Vassileva G, Disa J, Tadin-Strapps M, Stone DJ. TMEM175 deficiency impairs lysosomal and mitochondrial function and increases α-synuclein aggregation. Proc Natl Acad Sci U S A. 2017 Feb 28;114(9):2389-2394. Epub 2017 Feb 13 PubMed.
Further Reading
Primary Papers
- Hu M, Li P, Wang C, Feng X, Geng Q, Chen W, Marthi M, Zhang W, Gao C, Reid W, Swanson J, Du W, Hume RI, Xu H. Parkinson’s disease-risk protein TMEM175 is a proton-activated proton channel in lysosomes. Cell, June 23, 2022 Cell
- Zheng W, Shen C, Wang L, Rawson S, Xie WJ, Nist-Lund C, Wu J, Shen Z, Xia S, Holt JR, Wu H, Fu TM. pH regulates potassium conductance and drives a constitutive proton current in human TMEM175. Sci Adv. 2022 Mar 25;8(12):eabm1568. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
National Institute on Aging
The report by Hu et al. suggesting that TMEM175, a risk gene for PD, is a lysosomal proton channel is quite striking in the context of prior claims of activity encoded by this channel.
For example, work from Wie et al. (Wie et al., 2021) last year reported an AKT-gated K+ channel for the same gene product. As both papers use neuronal lysosomes, the apparent difference between the two claimed activities would not readily be ascribed to cell type. Hu et al. also report, at least in heterologous overexpression systems, that AKT does not affect TMEM175 activity, leading to an open question about which of the proposed activities is most reliable. However, both studies agree that TMEM175 deficiency increases synuclein pathology, here using striatal slices.
What would be important to further resolve is the effect of the TMEM175 p.M393T and Q65P variants at endogenous levels in neurons. Both of these studies, and prior reports such as Jinn et al., 2019, argue that TMEM175 p.M393T are certainly loss of function and possibly exert a dominant-negative effect over wild-type protein. Hence, we might expect neurons expressing this variant in the heterozygous state to behave like full knockouts in each of the proposed activity assays and in measures of synuclein pathology. Such experiments would be particularly instructive in the endogenous human context, which could feasibly be addressed by engineering in IPSC lines.
References:
Wie J, Liu Z, Song H, Tropea TF, Yang L, Wang H, Liang Y, Cang C, Aranda K, Lohmann J, Yang J, Lu B, Chen-Plotkin AS, Luk KC, Ren D. A growth-factor-activated lysosomal K+ channel regulates Parkinson's pathology. Nature. 2021 Mar;591(7850):431-437. Epub 2021 Jan 27 PubMed. Correction.
Jinn S, Blauwendraat C, Toolan D, Gretzula CA, Drolet RE, Smith S, Nalls MA, Marcus J, Singleton AB, Stone DJ. Functionalization of the TMEM175 p.M393T variant as a risk factor for Parkinson disease. Hum Mol Genet. 2019 Oct 1;28(19):3244-3254. PubMed.
Ohio State University
The major discovery of this paper is that TMEM175 is a proton channel in lysosomes, a longstanding and exceptionally important question in the field of lysosomal biology. I am glad to see that the Cell paper confirmed our discovery, which was published in Science Advances in March this year.
Our group first cracked this problem three years ago, and we submitted our manuscript covering mechanisms of both potassium conductance and proton conductance to Cell in March 2020. The review process took more than four months, and one reviewer rejected our manuscript without providing comments. After that, we spent another six months to fully develop one of our findings that TMEM175 is a lysosomal proton channel. We communicated with Cell editors and resubmitted our revised manuscript in January 2021. After another four months, it was rejected without reasonable comments. After that, we tried other journals without success. Last fall, we submitted to Science Advances and got published earlier this year.
Reading this paper felt like I was reading my own. Our publishing experience reminded me of a Nobel laureate’s words: “When you make an important discovery, people first say the discovery is not important, and then say that you are not the first one who came up with the idea.”
Despite our experience, I should say that this Cell paper is beautiful. Key experiments proving that TMEM175 is a proton channel in lysosomes are almost the same as ours, but one difference is that Hu et al. did lysosomal recording, an experiment we planned but did not do. Another difference is that Hu et al. found arachidonic acid can promote both proton and potassium conductance of TMEM175, which our paper did not cover. Hu et al. also used mouse experiments to show that the proton activity of TMEM175 is critical for Parkinson’s disease pathogenesis, confirming our hypothesis. In contrast, the mechanisms of proton conductance by TMEM175, which were clearly revealed in our published paper, were not discussed here.
In summary, our discovery and a similar discovery in this paper represent a breakthrough in the lysosomal biology, ion channel biology, and neurodegeneration fields. As proton conductance by TMEM175 is tightly associated with Parkinson’s disease, we believe developing TMEM175 agonists will have a chance to treat PD caused by TMEM175 mutations.
University of Tübingen
German Center for Neurodegernative Diseases (DZNE))
TMEM175 has gained a lot of attention recently as a potential target for disease modification in PD. It had been originally identified as a lysosomal potassium channel involved in the regulation of lysosomal function. The gene is located under the Parkinson’s disease GWAS peak on chromosome 4p16.3. Interestingly, two coding variants in the TMEM175 gene (M393T increasing and Q65P decreasing PD risk) were identified that seem to explain much or all of the variance associated with this locus and that also modify the penetrance of another major PD risk gene, GBA1. This set of observations directly links the function of the TMEM175 lysosomal channel to PD risk.
The paper by Hu et al. now provides a shift of paradigm concerning the expected function of TMEM175 as a potassium transporter:
They demonstrate that under physiological conditions, i.e., acidic lysosomal pH, TMEM175 conductance is much stronger for protons than for potassium ions. This nominates this channel as the postulated LyPAP (lysosomal proton activated proton channel) responsible for the observed proton leak, which is needed to stabilize the lysosomal pH by counteracting the V-ATPase-mediated lysosomal proton influx. In case of over-acidification, proton conductance is increased, leading to an outflow of protons, while under less-acidic conditions, the channel closes, allowing the ATP-driven proton pump to re-establish an acidic working pH in the lysosome of ~4.5.
So far, it has been demonstrated that TMEM175 is regulated by binding the active form of AKT, thereby reacting to extracellular signals, like growth factors. In contrast, Hu et al. now show that the TMEM175 is a LyPAP, as its conductance is dependent on the intraluminal proton concentration. Furthermore, the authors could also identify allosteric effectors such as arachidonic acid and two other synthetic ligands, which are able to activate the LyPAP TMEM175.
In light of this paper, it is now possible and necessary to revisit previous studies, relating known risk or protective variants of TMEM175 to lysosomal function and disease mechanisms. The further characterization of the binding site and the mechanism of channel activity modulation by arachidonic acid or other molecules could result in the identification of novel disease-modifying targets for PD, which is a very exciting development.
Northwestern University
Northwestern University
Through a series of careful and thorough experiments, Hu and colleagues demonstrate that the PD-risk-associated protein TMEM175 acts to counter the vacuolar-type H+ ATPase (V-ATPase) in regulating the pH of lysosomes. In response to lysosomal hyperacidification, TMEM175 effluxes protons to achieve an optimal pH necessary for lysosomal hydrolase function.
Previous publications had asserted that TMEM175 was a non-canonical potassium channel. However, this paper found that at physiological lysosomal pH (4.5-5.0), TMEM175 is 100,000 times more permeable to protons than potassium, and only resembles a potassium channel at a luminal pH of 7.0. These data agree with a paper published in March that showed that at a pH of 7.4, TMEM175 is permeable to potassium, but as pH drops its permeability increases for H+ and decreases for potassium (Zheng et al., 2022). The converging findings of Hu et al. with Zheng et al., which the authors note was published while Hu et al. was in its final phase of review, strengthen the claims of both papers.
Among the compelling findings of the paper was that arachidonic acid (ArA) and the small-molecule drugs DCPIB and ML67-33 activate the release of luminal H+ into the cytosol. In contrast to a previous study (Wie et al., 2021), they did not find that AKT activation was necessary or sufficient for TMEM175- mediated H+ currents. These results should prove useful to researchers aiming to manipulate the action of TMEM175 experimentally.
Additionally, the notion that ArA can modulate TMEM175 activity could have potential relevance to any disease state in which there is an ArA imbalance. Future studies can assess whether the effects of ArA are generalizable to other polyunsaturated fatty acids, and whether the activation of TMEM175 by lipids may serve to couple lipase activity and lipid availability to general lysosomal function.
The notion that such a ubiquitously expressed protein with such an overarching impact on lysosomal function should specifically be implicated in Parkinson’s disease highlights the importance of lysosomal function to dopaminergic neuron viability. This of course matches the genetics of PD, where variants in multiple lysosomal genes including GBA1, CTSB, ATP6V0A1, and TMEM175 modify PD risk (Chang et al., 2017).
The work of this paper and others suggests that the function of each of the proteins encoded by these genes are intimately connected. For instance, glucocerebrosidase (GCase), the protein product of GBA1, exhibits optimal hydrolase activity at a pH of 4.7-5.5 (Karatas et al., 2020). This narrow pH range must be maintained by the opposing action of TMEM175 and the V-ATPase, of which ATP6V0A1 encodes a subunit. Failure of these two should result in the failure of GCase. Accordingly, TMEM175 KO cells have reduced GCase activity (Jinn et al., 2017).
In this paper, the authors demonstrated that without TMEM175, cathepsin B activity is reduced. A recent study found that variants in the CTSB locus that decrease mRNA expression of CTSB modify risk of developing GBA-associated PD and Lewy body dementia (Blauwendraat et al., 2020).
Together these findings raise the possibility that variants in TMEM175, ATP6V0A1, and CTSB, may impact the penetrance of GBA risk alleles, and phenocopy them in individuals without GBA risk alleles through their effect on WT GCase activity. The impact of variants in these genes in concert and independently warrants further investigation.
References:
Zheng W, Shen C, Wang L, Rawson S, Xie WJ, Nist-Lund C, Wu J, Shen Z, Xia S, Holt JR, Wu H, Fu TM. pH regulates potassium conductance and drives a constitutive proton current in human TMEM175. Sci Adv. 2022 Mar 25;8(12):eabm1568. PubMed.
Wie J, Liu Z, Song H, Tropea TF, Yang L, Wang H, Liang Y, Cang C, Aranda K, Lohmann J, Yang J, Lu B, Chen-Plotkin AS, Luk KC, Ren D. A growth-factor-activated lysosomal K+ channel regulates Parkinson's pathology. Nature. 2021 Mar;591(7850):431-437. Epub 2021 Jan 27 PubMed. Correction.
Chang D, Nalls MA, Hallgrímsdóttir IB, Hunkapiller J, van der Brug M, Cai F, International Parkinson's Disease Genomics Consortium, 23andMe Research Team, Kerchner GA, Ayalon G, Bingol B, Sheng M, Hinds D, Behrens TW, Singleton AB, Bhangale TR, Graham RR. A meta-analysis of genome-wide association studies identifies 17 new Parkinson's disease risk loci. Nat Genet. 2017 Sep 11; PubMed.
Karatas M, Dogan S, Spahiu E, Ašić A, Bešić L, Turan Y. Enzyme kinetics and inhibition parameters of human leukocyte glucosylceramidase. Heliyon. 2020 Nov;6(11):e05191. Epub 2020 Nov 2 PubMed.
Jinn S, Drolet RE, Cramer PE, Wong AH, Toolan DM, Gretzula CA, Voleti B, Vassileva G, Disa J, Tadin-Strapps M, Stone DJ. TMEM175 deficiency impairs lysosomal and mitochondrial function and increases α-synuclein aggregation. Proc Natl Acad Sci U S A. 2017 Feb 28;114(9):2389-2394. Epub 2017 Feb 13 PubMed.
Blauwendraat C, Reed X, Krohn L, Heilbron K, Bandres-Ciga S, Tan M, Gibbs JR, Hernandez DG, Kumaran R, Langston R, Bonet-Ponce L, Alcalay RN, Hassin-Baer S, Greenbaum L, Iwaki H, Leonard HL, Grenn FP, Ruskey JA, Sabir M, Ahmed S, Makarious MB, Pihlstrøm L, Toft M, van Hilten JJ, Marinus J, Schulte C, Brockmann K, Sharma M, Siitonen A, Majamaa K, Eerola-Rautio J, Tienari PJ, 23andMe Research Team, Pantelyat A, Hillis AE, Dawson TM, Rosenthal LS, Albert MS, Resnick SM, Ferrucci L, Morris CM, Pletnikova O, Troncoso J, Grosset D, Lesage S, Corvol JC, Brice A, Noyce AJ, Masliah E, Wood N, Hardy J, Shulman LM, Jankovic J, Shulman JM, Heutink P, Gasser T, Cannon P, Scholz SW, Morris H, Cookson MR, Nalls MA, Gan-Or Z, Singleton AB. Genetic modifiers of risk and age at onset in GBA associated Parkinson's disease and Lewy body dementia. Brain. 2020 Jan 1;143(1):234-248. PubMed.
Make a Comment
To make a comment you must login or register.