Ubiquitin Peptidase Linked to Increased Tau Pathology in Women
Quick Links
Could overexpression of certain genes put women at a higher risk of Alzheimer’s disease? This seems to be the case for USP11, which partially evades silencing on one of the X chromosomes. This gene encodes a peptidase that removes ubiquitin chains from tau, which opens it up to acetylation instead, claim scientists led by David Kang and Jung-A Woo at Case Western Reserve University, Cleveland. This acetylation makes the protein more likely to aggregate, the authors concluded. In the September 22 Cell, the researchers reported that knocking out USP11 protected female mice from tauopathy. They found that USP11 levels tracked with neurofibrillary tangle burden in women, but not in men.
“This exciting finding links female-specific biology to tau,” wrote Dena Dubal, University of California, San Francisco. She noted that studies are increasingly uncovering a critical role for the X chromosome in brain aging and in Alzheimer’s disease. Li Gan of Weill Cornell Medicine, New York, who co-authored this paper, agreed. “The study highlights the need to consider sex differences in mechanisms underlying neurodegenerative diseases,” she wrote (comments below).
Because women have two X chromosomes, one must be silenced to avoid duplicate expression. However, some genes can evade this X-inactivation. “Up to a third of X chromosome genes are typically expressed on both the activated and inactivated X chromosome, and the degree of genetic expression varies across individuals,” noted Andrew Pieper of Case Western. Pieper is a co-author on the Cell paper. For example, Dubal previously pegged overexpression of another slippery X-gene, Kdm6a, as protective against AD (Aug 2020 news).
First author Yan Yan and colleagues came upon USP11 when they screened the 22 known de-ubiquitinases in the central nervous system for modulators of tau. Yan found two hits—USP11 and USP13. Because the USP11 gene sits on the X chromosome, the scientists wondered if it might help explain why women are more susceptible to tau pathology. To find out, they began by knocking down USP11 in HEK cells expressing P301L mutant tau. This halved tau concentration without changing its mRNA level, and reduced acetylation of lysine 281 in tau’s microtubule-binding domain. In contrast, overexpressing the enzyme increased the amount of insoluble, but not soluble, tau. What was going on?
The researchers found that USP11 chopped ubiquitin from 20 lysine residues, exposing them to acetylation. Tau acetylated at lysine 281 (K281Ac) was three times as insoluble as wild-type tau, they found, and acetylated tau is prone to aggregation, explaining how USP11 could keep that pathology in check (Mar 2011 news; Sep 2015 news; Trzeciakiewicz et al., 2017).
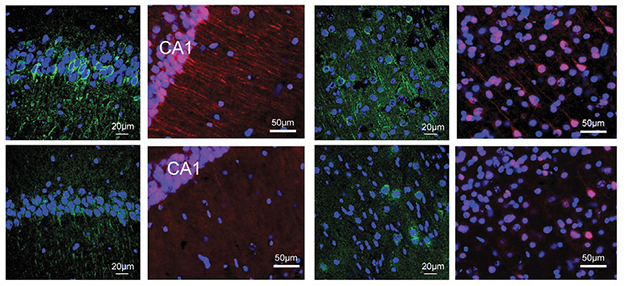
No USP11, Less P-tau. Hippocampal (left two columns) and cortical (right two columns) tissue from female USP11 knockout/P301S mice (bottom) had less ptau-202 (green) and less ptau262 (red) than did P301S mice (top). [Courtesy of Yan et al., Cell, 2022.]
These shenanigans held true in vivo, also, but there was a sex difference. Female mice carrying P301S tau had two to three times as much cortical USP11 and K281Ac tau as did males, in keeping with the idea that the gene evades X inactivation. Compared to wild-type female mice, 7-month-old P301S females had more soluble and insoluble tau, poorer long-term potentiation, and less synaptophysin, a synaptic vessel protein. They also had trouble finding a hidden platform in a water maze. Knocking out USP11 in the P301S females reduced soluble and insoluble total tau by 25 and 60 percent, respectively, soluble and insoluble K281Ac tau by 35 and 80 percent, and phospho-tau species by half (see image above). Synaptic signaling and memory were normalized. In contrast, knocking out USP11 had little effect in male P301S mice. These results suggest that loss of USP11 reduces tau pathology and restores synaptic plasticity and memory only in female mice.

Stronger in Women. USP11 and tau load correlated more tightly in women (left) who had had AD (top) or FTD (bottom) than in men (right). [Courtesy of Yan et al., Cell, 2022.]
This sex dichotomy was also apparent in people. The researchers analyzed frontal gyrus samples from 17 donors who had had AD, seven who had had frontotemporal dementia with tau pathology (FTD-tau), and 10 controls from the Emory University AD Research Center. Donors were 40 to 94 years old when they died, and half were female. Healthy women had three times more USP11 than did healthy men.
As a group, the people who had had AD or FTD had 9.5- and 6.5-fold more of the enzyme in the brain samples, respectively, than did controls. While USP11 co-localized with AT8-positive tangles in both men and women, this was much stronger in women (see image above). “The correlation of USP11 with tau pathology in female (not male) AD brains is striking,” Gan wrote. “It suggests that the moderate elevation of USP11, and the resulting increased tau acetylation, plays a critical role in tipping the balance of ubiquitination and acetylation that results in tau accumulation.”
All told, the researchers believe that inhibiting USP11, and therefore tau deubiquitination, might be a therapeutic strategy to help women stave off AD and other tauopathies.—Chelsea Weidman Burke
References
News Citations
- Does Second X Chromosome Boost Women’s Resilience Against Alzheimer’s?
- Tau Modification—Move Over Phosphate, Make Room for Acetylation
- New Type of Toxic Tau? Acetylated Form Correlates With Memory Defects
Mutations Citations
Research Models Citations
Paper Citations
- Trzeciakiewicz H, Tseng JH, Wander CM, Madden V, Tripathy A, Yuan CX, Cohen TJ. A Dual Pathogenic Mechanism Links Tau Acetylation to Sporadic Tauopathy. Sci Rep. 2017 Mar 13;7:44102. PubMed.
Further Reading
Papers
- Shin MK, Vázquez-Rosa E, Koh Y, Dhar M, Chaubey K, Cintrón-Pérez CJ, Barker S, Miller E, Franke K, Noterman MF, Seth D, Allen RS, Motz CT, Rao SR, Skelton LA, Pardue MT, Fliesler SJ, Wang C, Tracy TE, Gan L, Liebl DJ, Savarraj JP, Torres GL, Ahnstedt H, McCullough LD, Kitagawa RS, Choi HA, Zhang P, Hou Y, Chiang CW, Li L, Ortiz F, Kilgore JA, Williams NS, Whitehair VC, Gefen T, Flanagan ME, Stamler JS, Jain MK, Kraus A, Cheng F, Reynolds JD, Pieper AA. Reducing acetylated tau is neuroprotective in brain injury. Cell. 2021 May 13;184(10):2715-2732.e23. Epub 2021 Apr 13 PubMed.
- Caballero B, Bourdenx M, Luengo E, Diaz A, Sohn PD, Chen X, Wang C, Juste YR, Wegmann S, Patel B, Young ZT, Kuo SY, Rodriguez-Navarro JA, Shao H, Lopez MG, Karch CM, Goate AM, Gestwicki JE, Hyman BT, Gan L, Cuervo AM. Acetylated tau inhibits chaperone-mediated autophagy and promotes tau pathology propagation in mice. Nat Commun. 2021 Apr 14;12(1):2238. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Weill Cornell Medicine
The study provides a strong confirmation that the interplay between acetylation and ubiquitination modulates tau homeostasis, and it highlights the need to consider sex differences in targeting major mechanisms underlying neurodegenerative diseases quantitatively (Min et al., 2010; Min et al., 2015).
The correlation between USP11 and tau pathology in female (not male) AD brains is striking. It suggests that the moderate elevation of USP11 levels and resulting increased tau acetylation plays a critical role in tipping the balance of ubiquitination and acetylation that results in tau accumulation. Thus, restoring the balance to facilitate ubiquitination, either by inhibiting USP11, as shown in the current study, or inhibiting acetylation, as shown in our previous study (Min et al., 2015), could be of particular benefit for female tauopathy patients.
Usp11 is also engaged in a feedback loop with estrogen, highlighting that gonads and sex chromosomes could interact to jointly generate sex dimorphisms. In another words, the level of USP11 in females could result from a degree of escape from X-linked inactivation and from hormonal regulation. In addition, X inactivation also differs with age in hematopoietic stem cells, highlighting the need to understand age-dependent mechanisms of X-linked regulation.
By generating transgenic mice expressing constitutive acetylated forms of tau in both males and females, our earlier study established the importance of acetylated K274 and acetylated K281 in modulating synaptic plasticity (Tracy et al., 2016). I was surprised to find that the levels of insoluble ac-K274 and ac-K281 are significantly higher in female versus male PS19 mice. Female PS19 mice often have milder cognitive deficits in our hands. There might be unidentified protective mechanisms at play in these animals. In addition, this study also suggests that it would be worthwhile to examine how inhibitors of tau acetylation could affect tau-mediated neuronal/synaptic deficits in a sex-specific manner.
References:
Min SW, Chen X, Tracy TE, Li Y, Zhou Y, Wang C, Shirakawa K, Minami SS, Defensor E, Mok SA, Sohn PD, Schilling B, Cong X, Ellerby L, Gibson BW, Johnson J, Krogan N, Shamloo M, Gestwicki J, Masliah E, Verdin E, Gan L. Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat Med. 2015 Oct;21(10):1154-62. Epub 2015 Sep 21 PubMed.
Min SW, Cho SH, Zhou Y, Schroeder S, Haroutunian V, Seeley WW, Huang EJ, Shen Y, Masliah E, Mukherjee C, Meyers D, Cole PA, Ott M, Gan L. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron. 2010 Sep 23;67(6):953-66. PubMed.
Tracy TE, Sohn PD, Minami SS, Wang C, Min SW, Li Y, Zhou Y, Le D, Lo I, Ponnusamy R, Cong X, Schilling B, Ellerby LM, Huganir RL, Gan L. Acetylated Tau Obstructs KIBRA-Mediated Signaling in Synaptic Plasticity and Promotes Tauopathy-Related Memory Loss. Neuron. 2016 Apr 20;90(2):245-60. Epub 2016 Mar 31 PubMed.
Case Western Reserve University
It has long been recognized that women are more vulnerable to Alzheimer’s disease and other forms of tauopathy than men, but it has not been fully understood why. This work from the Woo and Kang laboratories shows that a basis for this phenomenon is higher expression in the female brain of the X-linked gene that encodes ubiquitin-specific peptidase 11 (USP11). This results in reduced tau clearance from the brain, as well as increased tau acetylation, leading to its pathological aggregation.
Because pathological accumulation of tau causes disease, Woo and Kang conducted an unbiased screen to look for new causes of tau pathology by identifying enzymes capable of removing the ubiquitin degradation signal from tau. The surprise was when they identified a protein (USP11) that was encoded on the X chromosome and subject to only partial X inactivation.
X chromosome inactivation occurs on one of the two X chromosomes in females to balance expression between XX females and XY males. However, the process of X chromosome inactivation is incomplete, and up to 1/3 of X chromosome genes are expressed on both the activated and inactivated X chromosomes. The degree of escape from inactivation varies across individuals. When a gene escapes inactivation, its expression is controlled by the same processes that normally control its expression on the non-inactivated X chromosome. Thus, female have higher brain levels of USP11 than males.
This work predicts that USP11-inhibiting medicines would be expected to protect women from developing the accelerated tau pathology that they exhibit relative to men, and thereby mitigate their enhanced vulnerability to Alzheimer’s disease.
Make a Comment
To make a comment you must login or register.