Human Microglia Make Themselves at Home in Mouse Brain
Quick Links
As microglia become more central to Alzheimer’s research, researchers are seeking better model systems to study their in vivo behavior. In culture, the cells rapidly alter their gene expression, and mouse microglia respond differently to disease than do their human counterparts. Chimeric mice may provide a solution. At the AD/PD meeting held March 27–31 in Lisbon, Portugal, two groups reported that human microglia transferred to mouse brain appeared to maintain their human identity (Apr 2019 conference news).
- Human microglia retain human gene expression profiles in mouse brain.
- They monitor their environment and activate in response to injury.
- They respond differently to amyloid than do mouse microglia.
Now, one of these groups has published their findings. In the July 30 Neuron, researchers led by Mathew Blurton-Jones at the University of California, Irvine, detail their chimeric mouse model, reporting that the transplanted microglia assume transcription states similar to those in human brain, surveil their surroundings, and respond appropriately to injuries and disease. In a mouse model of amyloidosis, human microglia had a distinct genetic response compared to mouse microglia. Researchers from Bart De Strooper’s lab at KU Leuven, Belgium, had reported very similar findings at ADPD. Their paper, currently under peer review, was posted to BioRχiv last February (Mancuso et al., 2019).
Other researchers expressed enthusiasm. “The work by Hasselmann et al. offers a powerful tool to better investigate human microglia during brain disease,” Marco Colonna and Simone Brioschi at Washington University in St. Louis wrote to Alzforum (full comment below). Oleg Butovsky at Brigham and Women’s Hospital, Boston, said this is the best approach to date for studying human microglia in vivo. “This is next-level, state-of-the-art work,” Butovsky said.
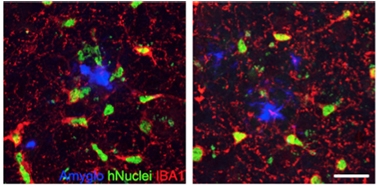
TREM2 Effect. In mouse cortex (left), wild-type human microglia (green with red processes) cluster around amyloid plaques (blue), but human TREM2 mutant microglia (right) do not. [Courtesy of Hasselmann et al., Neuron.]
To create the chimeric mouse, joint first authors Jonathan Hasselmann and Morgan Coburn transplanted fluorescently labeled hematopoietic progenitor cells derived from human iPSCs into the cortices and lateral ventricles of newborn transgenic MITRG mice. These mice express a humanized form of the microglial growth factor CSF1, which is essential for microglial maintenance, and lack two immune factors required for rejection of foreign cells. Two months later, the human cells had differentiated into macrophages and microglia. Near the injection sites, about 80 percent of microglia had a human, rather than mouse, pedigree. The authors found that the gene expression profiles of these xenotransplanted microglia closely matched those of human microglia the authors isolated from brain surgery samples, as well as a previously studied ex vivo microglia (Gosselin et al., 2017). The profiles differed from those of cultured human microglia, suggesting the transplanted cells better model in vivo microglial phenotypes.
The human cells appeared to behave normally in the mice. Through a cranial window, the authors observed the cells extending and retracting processes as they surveyed their environment. In response to an acute brain injury produced by a laser, nearby human microglia extended processes into the damaged region. After repeated mild head trauma, they moved into the damaged tissue and cleaned up neuronal debris. When mice were peripherally injected with lipopolysaccharide to stimulate an inflammatory response, the xenotransplanted cells dialed down homeostatic genes and turned up genes involved in phagocytosis and cytokine recognition. Notably, the response to LPS in vivo was distinct from that of cultured human microglia exposed to LPS, with almost no gene expression overlap.
To find out how human microglia would respond to amyloid, the authors crossed 5xFAD and MITRG mice, then transplanted human hematopoietic progenitor cells into the pups. At nine months of age, human microglia crowded around amyloid plaques. The cells had a rounded shape, and expressed numerous markers characteristic of disease-associated microglia (DAM), such as ApoE, TREM2, CD9, CD11C, and MERTK (Jun 2017 news). However, transcriptomic analysis revealed large differences between the DAM response in transplanted human and mouse microglia. Ninety percent of the changes in human microglia did not occur in the mouse cells; these comprised upregulation of 342 and downregulation of 336 genes. The set included AD risk genes such as MS4A6A, ABCG2, and CD33. The findings dovetail with previous research showing mouse and human microglia respond differently to amyloid (Feb 2018 news). The authors chose two of the human upregulated genes, HLA-DRB1 and LGALS3, and confirmed that both are highly expressed in microglia around amyloid plaques in human brain, suggesting the findings reflect what happens in AD.
This chimeric system could be used to study how AD mutations affect microglial responses, Blurton-Jones noted. As a proof of concept, the authors transplanted human microglia expressing the TREM2 R47H mutation into 5X-MITRG mice. The mutant cells poorly migrated to plaques, which is in keeping with findings from mouse models and human postmortem brain (see image above). The authors are examining the effects of other AD risk genes. Blurton-Jones noted that the model could also be used to study polygenic effects, by comparing microglial lines generated from people with high versus low cumulative genetic risk of AD.—Madolyn Bowman Rogers
References
News Citations
- Chimeric Mice: Can They Model Human Microglial Responses?
- Hot DAM: Specific Microglia Engulf Plaques
- Microglial Transcriptome Hints at Shortcomings of AD Model
Research Models Citations
Paper Citations
- Gosselin D, Skola D, Coufal NG, Holtman IR, Schlachetzki JC, Sajti E, Jaeger BN, O'Connor C, Fitzpatrick C, Pasillas MP, Pena M, Adair A, Gonda DD, Levy ML, Ransohoff RM, Gage FH, Glass CK. An environment-dependent transcriptional network specifies human microglia identity. Science. 2017 Jun 23;356(6344) Epub 2017 May 25 PubMed.
External Citations
Further Reading
Primary Papers
- Hasselmann J, Coburn MA, England W, Figueroa Velez DX, Kiani Shabestari S, Tu CH, McQuade A, Kolahdouzan M, Echeverria K, Claes C, Nakayama T, Azevedo R, Coufal NG, Han CZ, Cummings BJ, Davtyan H, Glass CK, Healy LM, Gandhi SP, Spitale RC, Blurton-Jones M. Development of a Chimeric Model to Study and Manipulate Human Microglia In Vivo. Neuron. 2019 Sep 25;103(6):1016-1033.e10. Epub 2019 Jul 30 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Washington University in St. Louis
Washington University School of Medicine
Some research groups were recently able to generate a surrogate of human microglia using induced-pluripotent stem cells (iPSC) derived from human fibroblasts (Muffat et al., 2016; Abud et al., 2017; Pandya et al., 2017; Takata et al., 2017). These studies were mostly carried out with in vitro co-culture systems, organtypic cultures, or brain organoids, which only partially recapitulate the complexity of the brain’s physiology in vivo. Indeed, modelling human microglia in an in vivo-like setting still represents a technical challenge.
Here, Hasselmann and colleagues transplanted human iPSC-derived hematopoietic progenitor cells (HPCs) into the brains of newborn immunodeficient mice. After transplantation, human HPCs spread throughout the mouse brain and successfully differentiate into microglia-like cells. Importantly, these cells exhibit a gene-expression profile resembling human microglia from surgical brain specimens. Moreover, IPSC-derived human microglia become readily activated upon acute inflammatory challenges, such as laser-induced cortical lesion or systemic LPS injection. In a mouse model of amyloid pathology, iPSC-derived human microglia efficiently migrate toward amyloid plaques and exhibit increased expression of immune-related genes (especially APOE, HLA-DR, MAFB, LGALS3, MS4A7, ITGAX, and TREM2).
Interestingly, iPSC-derived human microglia harboring the R47H TREM2 mutation (a well-known risk allele for Alzheimer’s disease in humans) show reduced clustering around the amyloid plaques, as compared to their wild-type counterpart. These findings suggest that microglia in subjects with R47H polymorphism might be hyporesponsive to Aβ, thus conveying a greater risk of developing Alzheimer’s disease during senility. Lastly, some genes that were previously reported to be upregulated in mouse microglia during amyloid pathology (such as Tyrobp, Cst7, Clec7a, and Csf1) were not significantly changed in iPSC-derived human microglia. These data suggest that mouse and human microglia mount different immunological responses against amyloid pathology in vivo. To conclude, the work by Hasselmann et al. offers a powerful tool to better investigate human microglia during brain disease.
References:
Muffat J, Li Y, Yuan B, Mitalipova M, Omer A, Corcoran S, Bakiasi G, Tsai LH, Aubourg P, Ransohoff RM, Jaenisch R. Efficient derivation of microglia-like cells from human pluripotent stem cells. Nat Med. 2016 Nov;22(11):1358-1367. Epub 2016 Sep 26 PubMed.
Abud EM, Ramirez RN, Martinez ES, Healy LM, Nguyen CH, Newman SA, Yeromin AV, Scarfone VM, Marsh SE, Fimbres C, Caraway CA, Fote GM, Madany AM, Agrawal A, Kayed R, Gylys KH, Cahalan MD, Cummings BJ, Antel JP, Mortazavi A, Carson MJ, Poon WW, Blurton-Jones M. iPSC-Derived Human Microglia-like Cells to Study Neurological Diseases. Neuron. 2017 Apr 19;94(2):278-293.e9. PubMed.
Pandya H, Shen MJ, Ichikawa DM, Sedlock AB, Choi Y, Johnson KR, Kim G, Brown MA, Elkahloun AG, Maric D, Sweeney CL, Gossa S, Malech HL, McGavern DB, Park JK. Differentiation of human and murine induced pluripotent stem cells to microglia-like cells. Nat Neurosci. 2017 May;20(5):753-759. Epub 2017 Mar 2 PubMed.
Takata K, Kozaki T, Lee CZ, Thion MS, Otsuka M, Lim S, Utami KH, Fidan K, Park DS, Malleret B, Chakarov S, See P, Low D, Low G, Garcia-Miralles M, Zeng R, Zhang J, Goh CC, Gul A, Hubert S, Lee B, Chen J, Low I, Shadan NB, Lum J, Wei TS, Mok E, Kawanishi S, Kitamura Y, Larbi A, Poidinger M, Renia L, Ng LG, Wolf Y, Jung S, Önder T, Newell E, Huber T, Ashihara E, Garel S, Pouladi MA, Ginhoux F. Induced-Pluripotent-Stem-Cell-Derived Primitive Macrophages Provide a Platform for Modeling Tissue-Resident Macrophage Differentiation and Function. Immunity. 2017 Jul 18;47(1):183-198.e6. PubMed.
Massachusetts Institute of Technology (MIT)
Picower Institute of MIT
To further dissect the response to AD pathology, Hasselmann and collaborators used single-cell transcriptomic profiling and compared xenotransplanted microglia (xMG) isolated from aged control versus xMG from 5X-MITRG mice. Interestingly, xMG from both control and disease model mice showed similar cellular heterogeneity, presenting clusters of MHC class II cells, type I interferon responding cells, seemingly homeostatic cells, and a cell group resembling the previously reported murine-disease-associated microglia (DAM) (Keren-Shaul et al., 2017). The latter population, marked by CD9, TREM2, LPL, and ITGAX; seemed increased in relative cell number in 5X-MITRG mice. However, when comparing the expression profiles of DAM versus homeostatic cells in the 5X-MITRG animals, the authors found a substantial number of differentially expressed genes not previously found in the murine DAM, suggesting a gene signature unique to human xMGs.
The lack of global agreement with murine DAM is consistent with our recent findings upon analyzing the single-cell heterogeneity of postmortem cortical samples (Mathys et al., 2019). Although partially overlapping with mouse disease-associated (Keren-Shaul et al., 2017) and mouse late-response (Mathys et al., 2017) microglia, a human AD-pathology-associated microglial subpopulation identified in our study also showed distinctive signatures not observed in mouse models. Future direct comparisons of global single-cell transcriptomic profiles, beyond marker-gene-set overlap and aided by larger samples, will likely help clarify the particularities of AD-responsive human microglia, their complete repertoire of cell states, and their similarity with the heterogeneity produced in previous mouse and “xenocultured” mouse models.
References:
Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, Itzkovitz S, Colonna M, Schwartz M, Amit I. A Unique Microglia Type Associated with Restricting Development of Alzheimer's Disease. Cell. 2017 Jun 15;169(7):1276-1290.e17. Epub 2017 Jun 8 PubMed.
Mathys H, Adaikkan C, Gao F, Young JZ, Manet E, Hemberg M, De Jager PL, Ransohoff RM, Regev A, Tsai LH. Temporal Tracking of Microglia Activation in Neurodegeneration at Single-Cell Resolution. Cell Rep. 2017 Oct 10;21(2):366-380. PubMed.
Mathys H, Davila-Velderrain J, Peng Z, Gao F, Mohammadi S, Young JZ, Menon M, He L, Abdurrob F, Jiang X, Martorell AJ, Ransohoff RM, Hafler BP, Bennett DA, Kellis M, Tsai LH. Single-cell transcriptomic analysis of Alzheimer's disease. Nature. 2019 Jun;570(7761):332-337. Epub 2019 May 1 PubMed.
Make a Comment
To make a comment you must login or register.