Plasma P-tau217 Picks Up Plaques, Tangles, Future Decline
Quick Links
A rapidly growing number of association studies across cohorts and continents are all pointing to the same conclusion: Plasma phospho-tau217 is an accurate biomarker for amyloid plaques, and even neurofibrillary tangles. That’s the consensus emerging from the Clinical Trials on Alzheimer’s Disease conference, held November 29 through December 2 in San Francisco. Several researchers concluded that the blood marker pegged plaque- or tangle-positive people with about 90 percent accuracy. Plasma p-tau217 also identified cognitively intact or impaired adults who were slipping fast on memory tests over six years. Given these findings, many scientists agreed that trialists may soon be able to forgo a PET scan in favor of a blood draw to screen for amyloid- and tau-positive participants.
- Plasma p-tau217 detected plaques and tangles in cognitively normal and impaired adults.
- The marker also pegged people with rapid memory decline.
- Screening with p-tau217 may halve the number of trial participants needed.
Earlier this summer at the Alzheimer’s Association International Conference, scientists homed in on plasma p-tau217 and the Aβ42/40 ratio as the leading markers of amyloid plaques in people with memory problems from community cohorts (Aug 2022 conference news).
This discussion continued at CTAD, where researchers agreed that p-tau217 also detects plaques, and even tangles and future cognitive decline, in cognitively normal older adults.
Cross-Sectional Confluence
For starters, Robert Rissman of the University of California, San Diego, reported that plasma p-tau217 tracked closely with amyloid PET positivity in 1,085 cognitively intact adults aged 55 to 80 in the Ahead trial. Of those, 345 were amyloid-positive, meaning they scored more than 20 centiloids on PET scans.
Rissman's team sent a blood sample from each participant to C2N Diagnostics in St. Louis, which measured Aβ42/40, and the ratios of plasma p-tau217, or p-tau181, to tau not phosphorylated at these residues. Rissman said that using a ratio normalizes for the total concentrations of each epitope. Some researchers say that p-tau ratios may correct for marker variation caused by conconcomitant diseases, while others have been unable to reproduce the normalization effect of ratios (see Part 14 of this series).
All three plasma markers distinguished amyloid-positive from -negative people, Rissman reported, but p-tau217/tau217 showed the clearest separation. The area under the curve, a combined measure of sensitivity and specificity, clocked in at 0.91 out of a perfect 1.0 for p-tau217/tau217, followed by 0.86 for Aβ42/40 and 0.77 for p-tau181/tau181 (see image below).
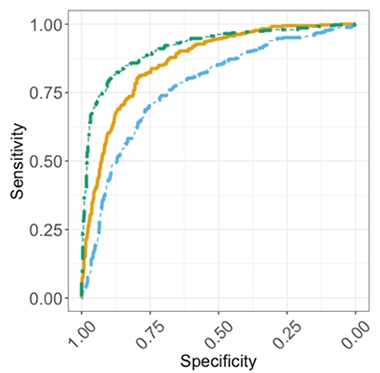
Accurate for Amyloid. Plasma p-tau217 (green) best pegged cognitively normal people who were amyloid-PET-positive, followed by Aβ42/40 (orange) and p-tau181 (blue). [Courtesy of Robert Rissman, UCSD.]
Combining the p-tau217 ratio with Aβ42/40, plus adding in the risk conferred by age and APOE status, nudged the AUC up to 0.95. Notably, the absolute concentration of either p-tau isoform alone identified amyloid-positive people almost as well as their p-tau/tau ratio, with AUCs of 0.90 and 0.74 for p-tau217 and p-tau181, respectively.
“This is getting to a level where we are very excited about using [these markers] in the community as screening for clinical trials,” Reisa Sperling of Brigham and Women’s Hospital, Boston, who directs the Ahead trial, said at the conference. Sperling and her husband, Keith Johnson of Massachusetts General Hospital, Boston, received the CTAD Lifetime Achievement Award in Alzheimer’s Disease Therapeutic Research for their contributions to AD clinical trials.
Just how soon does p-tau217 rise in blood once plaques begin accumulating in a person's brain? In Ahead participants, the marker starts to climb with as little as 11 centiloids of amyloid on their PET scans, Sperling said in her keynote.
Plasma p-tau217 tracked not only with amyloid PET. Johnson found that it also correlated with tau-PET positivity in the same cohort. In 303 amyloid-positive Ahead participants, Johnson measured plasma p-tau217/tau217 and p-tau181/tau181, amyloid PET, and tau PET. He focused on tangle load in the medial temporal allocortex (MTL) and the neocortex because these regions become PET-positive in the early and midstages of Alzheimer's tauopathy, respectively.
Johnson saw the same trend in marker correlations as did Rissman. Plasma p-tau217 most closely associated with MTL and neocortical tangles, giving correlation coefficients of 0.35 and 0.43 out of 1.0, respectively. Next came amyloid PET with coefficients of 0.33 and 0.27, then plasma p-tau181 at 0.12 and 0.20. Johnson concluded that high plasma p-tau217 might help identify tau-positive people who have preclinical AD.
Curious to see if p-tau217 even correlated with cognition, Johnson compared plasma markers and tau PET to scores on the Preclinical Alzheimer Cognitive Composite. Only the neocortical tau PET signal, not MTL tau load, amyloid PET, or p-tau level, associated with the PACC. This finding reinforces, in a different cohort, a finding by European researchers earlier this fall (Groot et al. 2022; Sep 2022 news).
Tracking Over Time
Could plasma p-tau217 predict a person's cognitive decline? Yes, according to multiple researchers showing their data at CTAD. Rebecca Langhough Koscik of the University of Wisconsin, Madison, studied 165 cognitively intact adults, average age 63, in the Wisconsin Registry for Alzheimer’s Prevention (WRAP) cohort. Over four years of follow-up, performance on the PACC remained steady in those with low plasma p-tau217 at baseline but fell off fast in those with high baseline plasma p-tau217, seven of whom developed MCI, and one dementia.
Koscik echoed Rissman and Johnson’s cross-sectional findings, reporting that plasma p-tau217 distinguished people who were amyloid or tau PET-positive at baseline with 91 or 95 percent accuracy, respectively.
For their part, Oskar Hansson and Niklas Mattsson-Carlgren at Sweden's Lund University reported that people with the highest plasma p-tau217 declined the fastest on a modified PACC and on the MMSE over six years (see image below). They studied 171 people with preclinical AD—i.e., those who are cognitively normal but test positive for plaques upon amyloid PET scan or CSF Aβ42/40 ratio—from the Swedish BioFINDER cohort and WRAP. The average ages were 73 and 64, respectively.
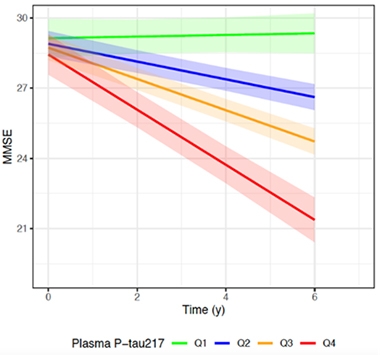
Faster Downhill. The higher a person’s baseline plasma p-tau217 concentration per quartile, the quicker his or her mental state slipped on the MMSE. [Courtesy of Oskar Hansson, Lund University.]
Of the 118 BioFINDER participants, 36 had progressed to AD dementia by their most recent follow-up visit. People in the highest quartile of plasma p-tau217 were twice as likely to progress to AD dementia as people in the lowest quartile. This analysis did not use the plasma p-tau217/tau217 ratio.
To learn which blood biomarker best foretold memory problems, Hansson compared baseline plasma p-tau181, p-tau217, p-tau231, glial fibrillary acidic protein, and neurofilament light concentrations to cognitive scores. P-tau217 best predicted cognitive decline, with correlation coefficients of 0.41 and 0.34 for mPACC and MMSE scores, respectively. P-tau181 clocked coefficients of 0.36 and 0.24, while GFAP came next with 0.30 and 0.13. NfL weakly correlated only with MMSE at 0.12, and plasma p-tau231 correlated with neither test.
This fits with a separate analysis of BioFINDER and WRAP participants, of whom 187 had mild cognitive impairment and 549 had normal cognition. Hansson, working with University of Gothenburg’s Kaj Blennow, found that plasma p-tau217—but not p-tau181, p-tau231, Aβ42/40, GFAP, or NfL—tracked with amyloid PET load, brain atrophy, and worsening mPACC and MMSE scores over six years (Ashton et al., 2022; Sep 2022 conference news). The scientists concluded that different plasma p-tau isoforms reflect different aspects of the disease process. Specifically, p-tau231 appears to rise at the earliest amyloid plaque accumulation, whereas p-tau217 seems to be a robust marker of progression.
A Stand-Up Finding Around the Globe
In Canada and Australia, plasma p-tau217’s predictive power held in cohorts of people who were already cognitively impaired. Just this month, Pedro Rosa-Neto of McGill University, Montreal, reported a strong correlation between plasma p-tau217 and amyloid PET—and to a lesser extent, tau PET—among 171 participants from the Canadian Translational Biomarkers in Aging and Dementia (Triad) cohort, who were staged across the AD clinical spectrum (Therriault et al., 2022).
Down under, Christopher Rowe, Austin Health, Melbourne, found similar results among 397 participants in the Australian Imaging, Biomarkers and Lifestyle (AIBL) and the Australian Dementia Network (ADNeT) cohorts (Doré et al., 2022).
Rowe used an assay developed by Janssen. Dubbed p217+tau, it is based on an antibody that binds to phosphorylated tau residues 217 and 212. In the AIBL and ADNeT cohorts, Rowe had found that plasma p217+tau tracked with amyloid and tau PET, giving AUCs of 0.89 and 0.85, respectively. Plasma p217+tau diagnoses AD equally well as p-tau217 does (Groot et al., 2022).
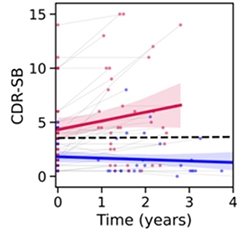
Worse Off. While cognition remained stable in people whose baseline plasma p217+tau was below 127 fg/mL (blue), CDR-SB scores started off higher and steadily worsened in people with high baseline p-tau217 (red). [Courtesy of Christopher Rowe, Austin Health.]
At CTAD, Rowe reported that plasma p217+tau also tracked with cognitive decline, as measured by MMSE and Clinical Dementia Rating-sum of boxes (CDR-SB), over an average of two years in 50 cognitively impaired AIBL participants. Half had MCI, half early stage AD. The blood marker predicted waning memory almost as well as did tau PET scans, and better than did amyloid scans. Cognition steadily worsened over three years in people who had high baseline plasma p217+tau but not in those with low levels (see image at right).
Taken together, all these studies suggest that plasma p-tau217 not only indicates who is amyloid-positive but also who is tau-positive and likely to suffer progressive cognitive decline over the next few years.
Good Enough for Trial Screening?
Could a simple blood draw to measure p-tau217 suffice to help trialists find amyloid-positive participants for investigative drug studies, then? Researchers are starting to think so. At CTAD, Rissman estimated that screening with plasma p-tau217/tau217 would halve the number of PET scans needed to enroll 1,000 amyloid-positive, cognitively normal people. Similarly, Hansson said that recruiting only cognitively intact people in the highest quartile for plasma p-tau217 could more than halve the sample size needed to see a statistically significant future decline via the mPACC and MMSE (see image below).
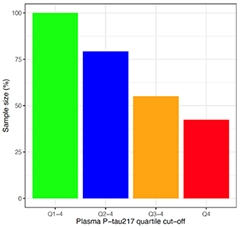
More P-tau, Fewer People. Compared to recruiting regardless of plasma p-tau217 concentration (green), enrolling only those with the highest levels (red) halved the number of participants needed to see differences in mPACC (shown) or MMSE scores (not shown) in this power calculation. [Courtesy of Oskar Hansson, Lund University.]
As for recruiting cognitively impaired people, Rowe cited cost savings of measuring plasma p217+tau. Assuming an amyloid or tau PET scan costs five times that of a blood test, he calculated an 80 percent savings of a trial's screening budget by switching to plasma p217+tau.
Apart from plaques, could plasma p-tau217 also find cognitively impaired people who have neurofibrillary tangles? Yes, said Gallen Triana-Baltzer of Janssen. He and colleagues prescreened people with MCI or early stage AD using plasma p217+tau for enrollment into the Auτonomy trial, a Phase 2 study testing the anti-tau antibody JNJ-63733657 in 420 cognitively impaired, tau PET-positive older adults, half of whom will have a low tangle burden. They found that most people with high p217+ also tested positive on tau PET.
Of 787 people screened from about 100 clinical sites across the U.S., Europe, and Asia, 72 percent were positive on the p217+tau blood test, based on a cutoff set to 100 fg/mL. Of those, 413 underwent a tau PET scan, and 87 percent were positive. However, two-thirds of those had a high tangle burden. To achieve the enrollment goal of equal numbers of people with low or high tangle burden, the scientists added an upper-limit cutoff of 250 fg/mL to eliminate those with highest p217+tau and hence highest tangle burden. Of an additional 504 people tested, 226 fell within the 100 to 250 fg/mL range, and 104 of those got a tau PET scan. Of 83 who tested positive, about half had a low tangle burden.
Triana-Baltzer concluded that plasma p217+tau can detect people who are tau-PET-positive, and that the marker rose along with tangle load. Screening out those with high levels of plasma p-217tau+ allowed them to achieve their goal of 50/50 low/high tangle burden.
Other assays for plasma p-tau217 include ALZpath. It uses an antibody developed by Abcam that was turned into a Simoa assay by Quanterix. In a CTAD poster, Stuart Portbury of ALZpath Bio in Carlsbad, California, presented the new assay's initial performance characteristics. In blood samples from 40 people with CSF-diagnosed AD, and 40 age- and sex-matched controls from a memory clinic, ALZpath distinguished AD with an AUC of 0.92. This was more accurate than Quanterix’s p-tau181 Simoa assay, which came in at 0.83. The signal was bigger, too: p-tau217 rose 4.2-fold in people with AD versus controls, compared to a 1.8-fold increase in p-tau181. ALZpath will be available for clinical use in early 2023, the company says.
Besides C2N, Janssen, and ALZpath, Lilly, Abbvie, Fujirebio, and other groups have, or are developing, blood tests for p-tau217. Blennow told Alzforum that all should be properly validated so their performance will be robust in clinical practice in different countries around the world. “There will be multiple plasma p-tau immunoassays on the market a few years from now,” Blennow said.—Chelsea Weidman Burke
References
News Citations
- Blood Tests Go Head-to-Head in Community Cohorts
- Blood Amyloid Test May Help Diagnose Alzheimer’s, but Questions Remain
- Scientists Say It's Time to Update ATN Criteria
- Head-to-Head Study Confirms Plasma p-Tau231 Rises First in Early AD
Paper Citations
- Groot C, Smith R, Stomrud E, Binette AP, Leuzy A, Wuestefeld A, Wisse LE, Palmqvist S, Mattsson-Carlgren N, Janelidze S, Strandberg O, Ossenkoppele R, Hansson O. Phospho-tau with subthreshold tau-PET predicts increased tau accumulation rates in amyloid-positive individuals. Brain. 2022 Sep 9; PubMed.
- Ashton NJ, Janelidze S, Mattsson-Carlgren N, Binette AP, Strandberg O, Brum WS, Karikari TK, González-Ortiz F, Di Molfetta G, Meda FJ, Jonaitis EM, Koscik RL, Cody K, Betthauser TJ, Li Y, Vanmechelen E, Palmqvist S, Stomrud E, Bateman RJ, Zetterberg H, Johnson SC, Blennow K, Hansson O. Differential roles of Aβ42/40, p-tau231 and p-tau217 for Alzheimer's trial selection and disease monitoring. Nat Med. 2022 Dec;28(12):2555-2562. PubMed.
- Therriault J, Vermeiren M, Servaes S, Tissot C, Ashton NJ, Benedet AL, Karikari TK, Lantero-Rodriguez J, Brum WS, Lussier FZ, Bezgin G, Stevenson J, Rahmouni N, Kunach P, Wang YT, Fernandez-Arias J, Socualaya KQ, Macedo AC, Ferrari-Souza JP, Ferreira PC, Bellaver B, Leffa DT, Zimmer ER, Vitali P, Soucy JP, Triana-Baltzer G, Kolb HC, Pascoal TA, Saha-Chaudhuri P, Gauthier S, Zetterberg H, Blennow K, Rosa-Neto P. Association of Phosphorylated Tau Biomarkers With Amyloid Positron Emission Tomography vs Tau Positron Emission Tomography. JAMA Neurol. 2023 Feb 1;80(2):188-199. PubMed.
- Doré V, Doecke JD, Saad ZS, Triana-Baltzer G, Slemmon R, Krishnadas N, Bourgeat P, Huang K, Burnham S, Fowler C, Rainey-Smith SR, Bush AI, Ward L, Robertson J, Martins RN, Masters CL, Villemagne VL, Fripp J, Kolb HC, Rowe CC. Plasma p217+tau versus NAV4694 amyloid and MK6240 tau PET across the Alzheimer's continuum. Alzheimers Dement (Amst). 2022;14(1):e12307. Epub 2022 Apr 5 PubMed.
- Groot C, Cicognola C, Bali D, Triana-Baltzer G, Dage JL, Pontecorvo MJ, Kolb HC, Osssenkoppele R, Janelidze S, Hansson O. Diagnostic and prognostic performance to detect Alzheimer's disease and clinical progression of a novel assay for plasma p-tau217. Alzheimers Res Ther. 2022 May 14;14(1):67. PubMed. Correction.
Other Citations
External Citations
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.