And Then There Were Five: CSF Proteomics Defines Alzheimer’s Subtypes
Quick Links
Proteins floating in the cerebrospinal fluid might do more than diagnose Alzheimer’s disease—they may identify different subtypes, according to researchers led by Betty Tijms of the Amsterdam University Medical Center and Pieter Jelle Visser at Maastricht University, both in the Netherlands. In a preprint uploaded to medRxiv on May 11, they reported that five unique subtypes of the disease emerged upon tallying about 1,060 proteins in the CSF of people with AD. Beyond a specific CSF proteome, a distinct pathology distinguished each type. These included overexcited neurons, an overactive immune system, leaky blood vessels, a dysfunctional choroid plexus, and dysregulated transcription. Subtypes also differed in genetic risk variant profile, degrees of brain atrophy, and clinical disease severity.
- CSF proteomics parsed AD into five subtypes.
- A distinct pathology defined each.
- They differed by brain atrophy, prevalence of AD genetic variants, and disease progression.
“This is undoubtedly a step forward in the molecular characterization of AD,” wrote Costantino Iadecola, Weill Cornell Medical College, New York (comment below). “The findings have critical therapeutic implications, as they support the concept that a ‘one size fits all’ therapy may no longer be tenable.”
While amyloid plaques and neurofibrillary tangles define AD, diverse cellular disturbances drive their deposition, muddying the etiological waters of this disease. To clarify its origins, Tijms and colleagues previously searched the CSF proteome for hints of pathophysiology, finding 705 proteins up- or downregulated among people with AD (Aug 2019 conference news; Tijms et al., 2020). The proteins fell into three ontology-defined subgroups that correlated with neuronal hyperplasticity, innate immune activation, and blood-brain barrier (BBB) dysregulation.
To cast a wider net, first author Tijms used mass spectrometry to analyze thousands of proteins within CSF samples from a separate cohort of 187 cognitively healthy older adults and 419 people with AD from the Alzheimer Center, Amsterdam. Participants with AD, defined by having low CSF Aβ42, ranged from preclinical to dementia. Tijms also assessed genetic variants, structural MRI scans, and clinical data to see how they related to proteomic changes.
Of the 3,863 proteins measured, 1,058 were either more or less abundant in people with AD. Tijms clustered these by whether they were up- or downregulated in sync, then used gene ontology to identify biological pathways associated with each cluster. Proteomic profiles suggested five subtypes based on cellular processes predicted to be dysfunctional: the three previously identified—neuronal hyperplasticity, innate immune activation, and BBB dysregulation—and two new ones, dubbed choroid plexus dysfunction and RNA dysregulation (image below).
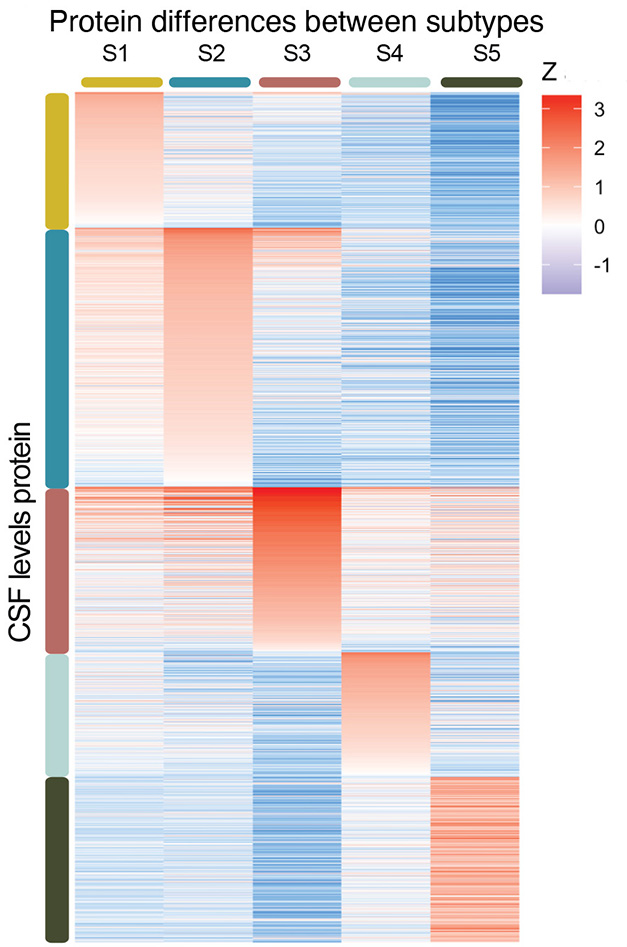
Five Flavors. In people with AD, the relative abundance of CSF proteins suggests five subtypes of the disease. [Courtesy of Tijms et al., medRxiv, 2023.]
Among the 419 people with AD, 137 fell into the neuronal hyperplasticity subtype. Upregulation of proteins involved in synapse assembly, axon guidance, and neuro- and gliogenesis suggested overactive neuron signaling and possibly an overabundance of neurons. Indeed, MRI scans showed the least atrophy in this subtype (image below). Only the hippocampus and temporal and parietal lobes shrank. Prevalence of the TREM2 R47H variant was highest in this group. This hypofunctional TREM2 hobbles microglial pruning of synapses in mouse models of amyloidosis and was recently linked to cortical synapse growth (Sep 2023 news). This subtype represented the mildest disease, with people living nine years, on average, after being clinically diagnosed with dementia.
In people with neuronal hyperplasticity, phosphotau-181, total tau, BACE1, Aβ40, and the lysosomal protein PLD3 were abundant in CSF. These are all found within dystrophic neurites (Oct 2016 conference news; Sadleir et al., 2016; Dec 2022 news). Tijms believes that in failing to compact amyloid, sluggish microglia help create these swollen axons around plaques, leading to elevated CSF BACE1 and Aβ in this subgroup.
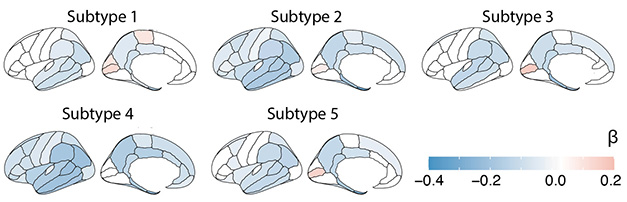
Atrophy by Subtype. Regional cortices thinned distinctly for each subtype of AD). [Courtesy of Tijms et al., medRxiv, 2023.]
Fifty-six people fit the BBB dysfunction criteria, having blood proteins, such as albumin, fibrinogens, and plasminogen, show up in the CSF. In contrast, there was a dearth of proteins made by brain vascular cells that typically leach into the CSF, such as platelet-derived growth factor receptor β and the cell adhesion proteins cadherin and laminin, suggesting disrupted brain tissue around blood vessels. Along these lines, people with the BBB dysfunction subtype had more microbleeds on MRI than people in other subtypes.
In contrast, microglia may be overactive in the second of the three previously identified subtypes, innate immune activation. Among the 124 people in this group, complement components, regulators of cytokine production, and microglial proteins were overrepresented. The latter included disease-associated microglial (DAM) markers ApoE and lipoprotein lipase, the cytokine CSF1, and its microglial receptor CSF1R, suggesting revved-up microglia. Tijms saw severe and widespread cortical atrophy in this group, perhaps because microglia prune synapses too vigorously, she speculated (image above; see Apr 2016 news). People with this subtype progressed the quickest from mild cognitive impairment to dementia.
People carrying common APP variants associated with AD were likelier to land in this BBB subtype (April 2022 news). Because APP duplications or the Dutch variant drive amyloid to deposit into the blood vessels of the brain, aka cerebral amyloid angiopathy, Tijms suspects other variants may be doing the same, compromising the BBB (Vervuurt et al., 2023).
As for the two new subtypes, molecules from the extracellular matrix and the choroid plexus (CP), including transthyretin, wound up in the CSF of 78 people with the CP subtype. MRI scans showed that the CP, a network of extracellular matrix and blood vessels, was enlarged. Large CPs associate with inflammation and cortical atrophy in multiple sclerosis, and in this fourth AD subtype, Tijms detected elevated cytokines and severe, widespread cortical thinning (Ricigliano et al., 2021; Chen et al., 2022).
People might have a genetic predisposition to this subgroup as well. They were likelier to carry AD risk variants in the molecular transporter ABCA7 and IL-34, both made by the choroid plexus, and in the endocytosis protein PICALM, which is expressed by vascular and perivascular cells that form the BBB. Evidence suggests that mutations in ABCA7 and in PICALM slow removal of Aβ from the brain, while IL-34 impairs microglia from clearing plaques (May 2015 news; Zuroff et al., 2020; Aug 2022 conference news). Unexpectedly, people with choroid plexus dysfunction had low levels of BACE1 and Aβ40 in the CSF, suggesting they don’t make as much Aβ. Taken together, these results suggested impaired clearance, rather than overproduction, of Aβ as the reason for amyloid accumulation in this type of AD.
The other new subtype, RNA dysregulation, comprised just 24 people. They had high levels of chaperones and RNA-binding proteins in their CSF. Intriguingly, they had little of the microtubule-binding protein stathmin-2. Correct splicing and translation of STMN2 requires the RNA-binding protein TDP-43, best known for its role in frontotemporal dementia. “While RNA dysfunction has been mainly observed in FTD, we now find that these disruptions are associated with a specific AD subtype as well,” noted Tijms and colleagues. TDP-43 fibrils also accumulate in AD.
This RNA dysregulation subtype seems the most aggressive. People had the most total tau and neurofilament light in their CSF, both signs of neuron damage, and they died soonest, about 5.5 years on average, after a clinical dementia diagnosis.
What implications might these subtypes have for AD therapies? “The associations between AD proteomic subtype and disease duration are relevant to the management of AD patients, both at the prognostic and therapeutic levels,” noted Iadecola. Likewise, Berislav Zlokovic, Ruslan Rust, and Marcelo Coba, all at the University of Southern California in Los Angeles, wondered if the subtypes responded differently to AD treatments (comment below). Tijms and Visser plan to test that by analyzing CSF samples from completed clinical studies.
Tijms and colleagues envision scientists using the subtypes to guide trial enrollment or therapy decisions. For example, they suggest TREM2-activating treatments to help people with the neuronal hyperplasticity subtype and some type of immune inhibitors for the innate immune activation subgroup.—Chelsea Weidman Burke
References
News Citations
- Proteomics Uncovers Potential Markers, Subtypes of Alzheimer’s
- In Alzheimer Brain, Can Synaptic Pruning Be Good?
- Does BACE Drive Neurites into Dystrophy, Shorting Circuits?
- Dystrophic Neurites Dampen Long-Range Neuronal Signaling
- Paper Alert: Microglia Mediate Synaptic Loss in Early Alzheimer’s Disease
- Paper Alert: Massive GWAS Meta-Analysis Published
- New Role For PICALM: Flushing Aβ From the Brain
- Rare Variants Net New Alzheimer’s Gene, Links Between AD and CAA
Mutations Citations
Mutation Position Table Citations
Paper Citations
- Tijms BM, Gobom J, Reus L, Jansen I, Hong S, Dobricic V, Kilpert F, Ten Kate M, Barkhof F, Tsolaki M, Verhey FR, Popp J, Martinez-Lage P, Vandenberghe R, Lleó A, Molinuevo JL, Engelborghs S, Bertram L, Lovestone S, Streffer J, Vos S, Bos I, Alzheimer’s Disease Neuroimaging Initiative (ADNI), Blennow K, Scheltens P, Teunissen CE, Zetterberg H, Visser PJ. Pathophysiological subtypes of Alzheimer's disease based on cerebrospinal fluid proteomics. Brain. 2020 Dec 1;143(12):3776-3792. PubMed.
- Sadleir KR, Kandalepas PC, Buggia-Prévot V, Nicholson DA, Thinakaran G, Vassar R. Presynaptic dystrophic neurites surrounding amyloid plaques are sites of microtubule disruption, BACE1 elevation, and increased Aβ generation in Alzheimer's disease. Acta Neuropathol. 2016 Aug;132(2):235-56. Epub 2016 Mar 18 PubMed.
- Vervuurt M, de Kort AM, Jäkel L, Kersten I, Abdo WF, Schreuder FH, Rasing I, Terwindt GM, Wermer MJ, Greenberg SM, Klijn CJ, Kuiperij HB, Verbeek MM. Decreased ratios of matrix metalloproteinases to tissue-type inhibitors in cerebrospinal fluid in sporadic and hereditary cerebral amyloid angiopathy. Alzheimers Res Ther. 2023 Jan 30;15(1):26. PubMed.
- Ricigliano VA, Morena E, Colombi A, Tonietto M, Hamzaoui M, Poirion E, Bottlaender M, Gervais P, Louapre C, Bodini B, Stankoff B. Choroid Plexus Enlargement in Inflammatory Multiple Sclerosis: 3.0-T MRI and Translocator Protein PET Evaluation. Radiology. 2021 Oct;301(1):166-177. Epub 2021 Jul 13 PubMed.
- Chen X, Luo D, Zheng Q, Peng Y, Han Y, Luo Q, Zhu Q, Luo T, Li Y. Enlarged choroid plexus related to cortical atrophy in multiple sclerosis. Eur Radiol. 2023 Apr;33(4):2916-2926. Epub 2022 Dec 22 PubMed.
- Zuroff LR, Torbati T, Hart NJ, Fuchs DT, Sheyn J, Rentsendorj A, Koronyo Y, Hayden EY, Teplow DB, Black KL, Koronyo-Hamaoui M. Effects of IL-34 on Macrophage Immunological Profile in Response to Alzheimer's-Related Aβ42 Assemblies. Front Immunol. 2020;11:1449. Epub 2020 Jul 16 PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Tijms BM, Vromen EM, Mjaavatten O, Holstege H, Reus LM, vanderLee SJ, Wesenhagen K, Lorenzini L, Vermunt L, Venkatraghavan V, Tesi N, Tomassen J, denBraber A, Goossens J, Vanmechelen E, Barkhof F, Pijnenburg YA, vanderFlier WM, Teunissen CE, Berven F, Visser PJ. Large-scale cerebrospinal fluid proteomic analysis in Alzheimer's disease patients reveals five molecular subtypes with distinct genetic risk profiles. 2023 May 11 10.1101/2023.05.10.23289793 (version 1) medRxiv.
Follow-On Reading
Papers
- Tijms BM, Vromen EM, Mjaavatten O, Holstege H, Reus LM, van der Lee S, Wesenhagen KE, Lorenzini L, Vermunt L, Venkatraghavan V, Tesi N, Tomassen J, den Braber A, Goossens J, Vanmechelen E, Barkhof F, Pijnenburg YA, van der Flier WM, Teunissen CE, Berven FS, Visser PJ. Cerebrospinal fluid proteomics in patients with Alzheimer's disease reveals five molecular subtypes with distinct genetic risk profiles. Nat Aging. 2024 Jan;4(1):33-47. Epub 2024 Jan 9 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Weill College Medicine, New York
This is undoubtedly a step forward in the molecular characterization of AD, which, in concert with pathological and imaging studies, indicates that the molecular drivers are diverse, even though amyloid and tau may be frequent downstream offenders. The findings have critical therapeutic implications because they support the concept that a “one-size-fits-all” therapy may no longer be tenable, and that treatment approaches for AD need to be guided by the underlying molecular drivers on a patient-by-patient basis.
As in similar studies, the mechanisms suggested by proteomic analysis need to be independently validated at the functional level. In this regard, it would be desirable to have blood proteomics at the time of CSF collection. This would have provided some insight into the source of the proteins differentially represented in AD patients compared to controls. This issue is particularly relevant to the proteins observed in the group with presumed BBB dysfunction, in which some proteins were assumed to have crossed the BBB and reached the CSF, but the possibility of endogenous synthesis cannot be excluded.
The statement about amyloid clearance and CAA also needs to be independently validated by imaging/pathological approaches. It would also have been valuable to examine which of the different AD subgroups has coexisting macro- and micro-vascular pathology. Intracranial atherosclerosis has been linked to RNA processing dysfunction and neuroplasticity (Wingo et al., 2020), while white-matter lesions may hint at BBB dysfunction (Rudilosso et al., 2023; Shirzadi et al., 2023). Despite these considerations the study is a step in the right direction in expanding the pathobiology of AD beyond Aβ and tau, and in suggesting new therapeutic strategies and targets. The findings implicating the choroid plexus are particularly intriguing, and provide support to imaging studies implicating alterations of this poorly understood structure in AD. Whether these proteomic alterations are a cause or an effect of the pathology remains to be established, but the finding will undoubtedly enhance the interest of the AD scientific community in the choroid plexus.
I also find the link between AD subtype and survival very interesting, as it provides insight into the aggressiveness of the pathology, being greatest for the RNA dysfunction subtype. These associations between AD proteomic subtype and disease duration are also relevant to the management of AD patients, at both the prognostic and therapeutic levels.
Overall, the findings support the usefulness of CSF proteomics in the phenotypic characterization of patients with AD. Whether this approach will remain a valuable research tool, or will be transferable to community health care, remains to be seen.
References:
Wingo AP, Fan W, Duong DM, Gerasimov ES, Dammer EB, Liu Y, Harerimana NV, White B, Thambisetty M, Troncoso JC, Kim N, Schneider JA, Hajjar IM, Lah JJ, Bennett DA, Seyfried NT, Levey AI, Wingo TS. Shared proteomic effects of cerebral atherosclerosis and Alzheimer's disease on the human brain. Nat Neurosci. 2020 Jun;23(6):696-700. Epub 2020 May 18 PubMed. Correction.
Rudilosso S, Stringer MS, Thrippleton M, Chappell F, Blair GW, Jaime Garcia D, Doubal F, Hamilton I, Janssen E, Kopczak A, Ingrisch M, Kerkhofs D, Backes WH, Staals J, Duering M, Dichgans M, Wardlaw JM, SVDs@target consortium. Blood-brain barrier leakage hotspots collocating with brain lesions due to sporadic and monogenic small vessel disease. J Cereb Blood Flow Metab. 2023 Sep;43(9):1490-1502. Epub 2023 May 3 PubMed.
Shirzadi Z, Schultz SA, Yau WW, Joseph-Mathurin N, Fitzpatrick CD, Levin R, Kantarci K, Preboske GM, Jack CR Jr, Farlow MR, Hassenstab J, Jucker M, Morris JC, Xiong C, Karch CM, Levey AI, Gordon BA, Schofield PR, Salloway SP, Perrin RJ, McDade E, Levin J, Cruchaga C, Allegri RF, Fox NC, Goate A, Day GS, Koeppe R, Chui HC, Berman S, Mori H, Sanchez-Valle R, Lee JH, Rosa-Neto P, Ruthirakuhan M, Wu CY, Swardfager W, Benzinger TL, Sohrabi HR, Martins RN, Bateman RJ, Johnson KA, Sperling RA, Greenberg SM, Schultz AP, Chhatwal JP, Dominantly Inherited Alzheimer Network and the Alzheimer’s Disease Neuroimaging Initiative. Etiology of White Matter Hyperintensities in Autosomal Dominant and Sporadic Alzheimer Disease. JAMA Neurol. 2023 Dec 1;80(12):1353-1363. PubMed.
University of Southern California
University of Southern California
USC
Alzheimer’s disease is molecularly highly heterogeneous, involving many different pathophysiological processes. This heterogeneity may also contribute to the limited effects observed in current clinical Alzheimer’s disease (AD) trials, highlighting the need for personalized treatment. Defining those molecular AD subtypes may be possible through analyzing cerebral spinal fluid (CSF), as CSF has been shown to carry a multifaceted AD signature that goes beyond Aβ and tau (Johnson et al., 2023; Del Campo et al., 2022).
This new, large-scale, CSF quantitative proteomic analysis from 609 individuals (AD: 419, CTRL: 187) identified a total of 3,863 proteins, of which approximately one-third was observed across all individuals. Proteins were further quantitated using 16 plex tandem mass tags (TMT), fractionated, and analyzed by state-of-the-art LC-MS/MS with FAIMS, and data dependent acquisition. Cluster analysis in AD individuals revealed three previously identified subtypes characterized by 1) neuronal hyperplasticity, 2) innate immune activation, and 3) BBB dysfunction, and two new subtypes: 4) RNA dysregulation and 5) choroid plexus dysfunction, all associated with distinct AD genetic risk factors.
The CSF proteomics for classifying AD subtypes may be a valuable resource in developing specific future treatments. For instance, patients exhibiting pronounced innate immune activation might respond more effectively to immune modulators. Conversely, those showing signs of neuronal hyperplasticity could potentially benefit from treatments that activate TREM2. Individuals with predominant vascular dysfunction and a subtype of AD with blood-brain barrier (BBB) breakdown that can predict cognitive decline might benefit from treatments preventing the BBB leaks into the brain and stabilizing the BBB integrity.
Future clinical studies will reveal whether the classified AD subtypes show different responses to particular treatments, or whether the classification can be used to select suitable subjects for upcoming clinical trials. Ideally, the presented AD subtype classification could be complemented by future studies with blood-based tests, addressing a broader AD population and facilitating earlier detection and therapeutic intervention in the disease process.
References:
Johnson EC, Bian S, Haque RU, Carter EK, Watson CM, Gordon BA, Ping L, Duong DM, Epstein MP, McDade E, Barthélemy NR, Karch CM, Xiong C, Cruchaga C, Perrin RJ, Wingo AP, Wingo TS, Chhatwal JP, Day GS, Noble JM, Berman SB, Martins R, Graff-Radford NR, Schofield PR, Ikeuchi T, Mori H, Levin J, Farlow M, Lah JJ, Haass C, Jucker M, Morris JC, Benzinger TL, Roberts BR, Bateman RJ, Fagan AM, Seyfried NT, Levey AI, Dominantly Inherited Alzheimer Network. Cerebrospinal fluid proteomics define the natural history of autosomal dominant Alzheimer's disease. Nat Med. 2023 Aug;29(8):1979-1988. Epub 2023 Aug 7 PubMed.
Del Campo M, Peeters CF, Johnson EC, Vermunt L, Hok-A-Hin YS, van Nee M, Chen-Plotkin A, Irwin DJ, Hu WT, Lah JJ, Seyfried NT, Dammer EB, Herradon G, Meeter LH, van Swieten J, Alcolea D, Lleó A, Levey AI, Lemstra AW, Pijnenburg YA, Visser PJ, Tijms BM, van der Flier WM, Teunissen CE. CSF proteome profiling across the Alzheimer's disease spectrum reflects the multifactorial nature of the disease and identifies specific biomarker panels. Nat Aging. 2022 Nov;2(11):1040-1053. Epub 2022 Nov 10 PubMed.
Emory University
This study employs unbiased clustering of over 400 CSF proteomic profiles to categorize or subtype distinct groups of individuals clinically diagnosed with AD. This type of unbiased classification is important because, despite displaying indications of dementia, or positivity for amyloid and/or tau biomarkers, individuals do not follow the same disease course. The authors identified five distinct subtypes of AD characterized by their CSF proteomic profiles and investigated their relationships with genetic risk and clinicopathologic phenotypes. These groups were classified based on biological ontologies that effectively differentiated them from one another. This classification included neuronal hyperplasticity, innate immunity, RNA dysregulation, choroid plexus dysfunction, and blood-brain barrier (BBB) dysfunction. Notably, some of the subtypes differed in their rate of progression from MCI to dementia and in brain atrophy.
It was intriguing that individuals within two of these subtypes, choroid plexus and BBB dysfunction, comprising approximately one-third of the cohort, displayed lower levels of tau and p-tau compared to the other three subtypes. This implies that mechanisms beyond tau may be influencing their cognitive decline, or alternatively, that tau is not an optimal biomarker for these subclasses. Genetic profiles were linked to subtypes, also, revealing an association with genetic risk, particularly in the hyperplasticity subtype, which is enriched with TREM2 variant carriers. The authors propose that individuals with this subtype may respond to treatments targeting TREM2, even if they do not carry the TREM2 R47H variant. It was also interesting that APOE4 carriers were enriched, albeit not statistically, in the BBB subtype compared to other subtypes, because APOE4 carriers are known to have increased cerebrovascular dysfunction compared to noncarriers.
One outstanding question is whether individuals within specific subtypes are more or less responsive to particular drug treatments. The authors propose that additional studies should aim to analyze or even retrospectively reanalyze CSF in clinical trials by proteomics. This would allow the assessment of whether specific treatments have effects that are specific to only certain subtypes. If this proves to be the case, it could pave the way for personalized medicine approaches in the treatment of AD.
Make a Comment
To make a comment you must login or register.