Does Brain Development in Childhood Set the Stage for Dementia?
Quick Links
It’s clear by now that in dementia, a disease mostly of old age, trouble starts decades earlier—but how early? Well—how about in the womb? Data presented at the Alzheimer's Association International Conference, 2015, held July 18-23 in Washington, D.C., lend some support to this radical idea. Scientists presented epidemiological, imaging, and even genetic studies that drew associations between early brain development and some atypical forms of Alzheimer's disease.
They also grappled with what those associations mean. “Might how your brain is wired in early stages influence how it degenerates later in life? That’s an extremely interesting concept,” said Jonathan Schott, University College London. Zachary Miller, from the University of California, San Francisco, stressed that early developmental patterns are not necessarily risk factors for later disease. "A more parsimonious explanation is that if you are going to get some form of dementia, then where it presents first, or the ‘locus of least resistance,’ might be determined by how your brain has developed," he told Alzforum. Whatever the cause and effect, researchers agreed that studying how learning disabilities and brain development influence later dementia could yield valuable insights into the disease processes, and even provide an opportunity for very early intervention.
Researchers have suspected for some time that there is a connection between learning problems and dementia. In 2008, researchers led by Marsel Mesulam at Northwestern University, Chicago, reported that people with learning disabilities, particularly dyslexia, were over-represented among patients with primary progressive aphasia (PPA), an atypical form of Alzheimer's disease characterized by language problems (see Feb 2008 news). Miller has studied this relationship, correlating learning disabilities with different forms of PPA and with posterior cortical atrophy (PCA), a form of dementia centered in the visuospatial cortex. He outlined in D.C. that screening 198 people in the PPA cohort at the UCSF Memory and Aging Center uncovered a connection between learning disability and the logopenic variant of PPA (lvPPA), but not with the semantic PPA or non-fluent variants (see Miller et al., 2013). Twelve of 48 people in the lvPPA group reported a history of learning disability, a prevalence as much as five times higher than that in the general population. Dementia started earlier in those 12 volunteers, hinting at particularly aggressive disease. Au contraire, MRI scans revealed their brain atrophy was much more restricted than in PPA patients with no history of learning problems, suggesting that neurodegeneration was more focal (see image below). In keeping with this idea, the learning disability/lvPPA patients scored higher on tests of global cognition than PPA patients in general. Miller's work suggests that early learning patterns might subtly influence the course of sub-types of dementia.
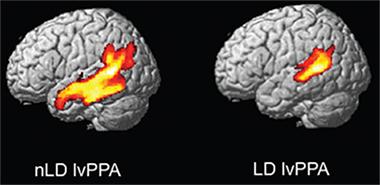
Deconstructing Aphasia.
Logopenic variant PPA patients with a history of learning disability have more focal atrophy (right). [Courtesy of Zachary Miller and Oxford University Press.]
Most of the patients with learning disability/PPA had childhood dyslexia, which predominantly causes reading difficulties. Could other learning disabilities, such as dyscalculia, or trouble with math, also herald PPA or some other kind of dementia?
Dyscalculia manifests as problems with numerosity, aka the ability to guess "more," as in which pile of acorns is bigger. The skill is conserved across most of the animal kingdom, said Miller. Without this basic math, animals would make poor decisions, e.g., squirrels might not survive the winter. In children, dyscalculia is the most common non-language learning disability. It can be inherited and has been linked to differences in brain structure and cortical activation patterns (see Kucian et al., 2006). Unlike language, which originates from the left side of the brain, the location of mathematical abilities is challenging to pin down. Symbolic mathematics typically localize to the left side, but non-verbal mathematical abilities tend to be rooted to the right side. Would people with dyscalculia be more likely to have neurodegeneration in the right brain hemisphere?
That's exactly what Miller found. In a UCSF cohort of 95 PCA patients, 17 had learning disabilities. Eleven of these had mathematical or visuospatial problems rather than language difficulties—a rate twice that in the general population. Interestingly, PCA patients with dyscalculia had atrophy predominantly in the right side of the brain, and much less in left side, compared with PCA patients without a history of learning disability. And similarly to the lvPPA patients with dyslexia, PCA patients with dyscalculia scored better on the MMSE and global cognition tests than did PCA patients without learning disabilities. Miller proposed that as for PPA, there may be sub-types of PCA with a more focal atrophy that might be distinguishable by brain imaging and natural history. Such subtypes might open a window onto the origin and progression of disease pathology. "The learning-disability groups may help us understand what is happening more generally in dementia," said Miller. Gil Rabinovici, who works with Miller at UCSF, agreed. "Trying to understand the mechanism that drives heterogeneity in dementia is incredibly important," he said. "There are fundamental clues among the rare variants that may teach us about vulnerability in general."
Researchers at the meeting asked if these results meant that people with learning disabilities were at greater risk for dementia. Miller said the available data is insufficient to draw that conclusion. He favored a "two-hit hypothesis" of how a person is built plus how he or she lives as determining overall risk. Others wondered why the atrophy in the cases with prior disability was so focal. Miller was unsure, but wondered whether it might be related to the structure of the brain in those regions or to some barrier to the spread of toxic tau.
Could genetic variants that cause or influence learning disabilities also predispose to dementia? Miller said there is as yet no clear evidence for this question, but it needs to be investigated. In his presentation, Schott described a variant that might do just that.
Schott led an international collaboration to carry out the most extensive genome-wide association study on PCA to date. Because the disease is so rare, the previously largest genetic analysis relied on about 80 patients. Schott and colleagues almost quadrupled that, obtaining DNA from 302 patients at 11 centers in Europe, North America, and Australia. "This might seem small for a genetics study, but it’s huge for PCA," said Schott. He credits the Atypical Alzheimer’s Disease and Associated Syndromes professional interest area organized through the Alzheimer's Association with helping pull together interested parties.
Patients recruited were 59 years old on average at symptom onset, which is typical for PCA. CSF, amyloid PET, or autopsy data was available for 82 of the volunteers; it was consistent with a diagnosis of Alzheimer's disease.
The researchers first looked in these DNA samples for genetic variants known to associate with AD. They confirmed that ApoE and CR1 variants were risk factors, though the odds ratio for ApoE was lower than for typical, late-onset AD. The researchers also detected nominal risk for ABCA7 and BIN1. In the GWAS, they identified three novel loci near the genes CNTNAP5, FAM46A, and SEMA3C that met genome-wide statistical significance. CNTNAP5 codes for a transmembrane protein involved in cell adhesion and signaling; FAM46A is expressed in the retina and has been linked to retinitis pigmentosa (see Barragán et al., 2008); and SEMA3C regulates axonal guidance in the developing brain.
Schott emphasized that these results are preliminary and need to be replicated. That said, he noted that FAM46A bears detailed study given the visual problems that plague patients with PCA, such as poor depth perception and difficulty tracking objects that may be related to reading difficulties. He is also intrigued by the notion that a gene involved in brain development—SEMA3C—could be linked to dementia. This gene codes for semaphorin-3C, one of a family of proteins that guide developing axons to the proper location. It is expressed in the visual cortex and the hippocampus, particularly near cholinergic afferents, said Schott. Interestingly, in a separate study SEMA3C emerged among 100 genes that supported connectivity among different brain networks, while SEMA3A, another semaphorin, was linked to control of axonal growth cones by β-secretase (see June 2015 news). "The possibility that subtle differences in brain development might influence the development of specific forms of dementia in later life is intriguing," said Schott. "If true, then this adds another dimension for study," he said. Schott noted that such developmental risks might get drowned out in studies of AD, but that by studying selective phenotypic variants of dementia, researchers might be able to lay bare those relationships.
What might vulnerabilities rooted in early life mean for the later treatment of dementia? "We could have 50 years lead time if we utilize the education system to identify learning disability," suggested Miller. In fact, a study in Sweden bears this out. At AAIC Serhiy Dekhtyar from the Karolinska Institute in Stockholm reported a link between test scores in primary school and dementia incidence later in life. Researchers in Sweden have followed 7,500 adults, age 65 and older, in the Uppsala Birth Cohort Study, for more than 20 years. Dekhtyar and colleagues correlated data on incident dementia with a variety of historical markers, including primary school grades, education level, and complexity of their occupation. The study is unique, said Dekhtyar, in having robust data on patient history. Though people in the cohort were born between 1915 and 1929, and school test records were archived and accessible. Dekhtyar obtained educational and occupational data from a population census taken around 1980, and dementia diagnoses from the national patient and cause-of-death register.
At AAIC, Dekhtyar reported that those who were in the lowest quintile for test scores at around age 10 had a 20 percent greater risk of developing dementia later in life. Interestingly, the association held even if the child went on to have many years of higher education or work in an occupation of higher complexity, both of which increase cognitive reserve. "It could be that the foundation for cognitive reserve begins very early in life," said Dekhtyar.
Dekhtyar noted that the Swedish registries tend to underestimate dementia diagnoses. To test if that might have biased the results, Hui-Xin Wang, also from the Karolinska, conducted a similar analysis on data from the Kungsholmen Study in Stockholm, which uses extensive clinical and neuropsychological assessment to diagnose AD. Among 440 men and women over age 75, those who had been in the lowest quintile for school grades at age 9-10 were 50 percent likelier to be diagnosed with dementia during the nine-year follow-up period. Neither study looked at specific types of dementia, but Dekhtyar said that data could be extracted from the records and agreed it would be interesting to see if test scores correlated with PPA, PCA, or other subtypes of dementia
While the Swedish studies did not specifically account for learning disabilities, Miller said they underscored the importance of a healthy brain in early life. "We need to think about lifespan neurology," said Miller. "It's important to understand people's entire life history. That influences who they are and their later vulnerabilities," he said.—Tom Fagan
References
News Citations
- Primary Progressive Aphasia—Learning Disabilities Point to Early Susceptibility
- Synaptic Genes Determine Brain Connectivity
Paper Citations
- Miller ZA, Mandelli ML, Rankin KP, Henry ML, Babiak MC, Frazier DT, Lobach IV, Bettcher BM, Wu TQ, Rabinovici GD, Graff-Radford NR, Miller BL, Gorno-Tempini ML. Handedness and language learning disability differentially distribute in progressive aphasia variants. Brain. 2013 Nov;136(Pt 11):3461-73. PubMed.
- Kucian K, Loenneker T, Dietrich T, Dosch M, Martin E, von Aster M. Impaired neural networks for approximate calculation in dyscalculic children: a functional MRI study. Behav Brain Funct. 2006 Sep 5;2:31. PubMed.
- Barragán I, Borrego S, Abd El-Aziz MM, El-Ashry MF, Abu-Safieh L, Bhattacharya SS, Antiñolo G. Genetic analysis of FAM46A in Spanish families with autosomal recessive retinitis pigmentosa: characterisation of novel VNTRs. Ann Hum Genet. 2008 Jan;72(Pt 1):26-34. Epub 2007 Sep 5 PubMed.
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.