Next Up for Human Brain Imaging: Synaptic Density?
Quick Links
Early in Alzheimer’s, connections between neurons begin to wither and die. One of the defining features of AD, this synapse loss underlies cognitive impairment, but has proven hard to track in the living brain. A paper in the July 20 Science Translational Medicine may change that. Scientists led by Richard Carson and Sjoerd Finnema at Yale University in New Haven, Connecticut, report on a positron emission tomography (PET) tracer that binds to a protein on presynaptic vesicles, revealing the density of synapses throughout the brain. In healthy people, the compound entered the brain quickly and bound predominantly gray matter. In epilepsy patients, it picked up areas of synaptic loss near the focal point of seizures.
“This paper shows what appears to be the first radioligand to bind to sites at synapses in the brain,” wrote Richard Mohs, Global Alzheimer’s Platform Foundation, to Alzforum. If confirmed, this could provide a way to measure synaptic density in living people and track changes in density regionally and with disease, he added. “These scans would complement other newly developed radioligands that allow imaging of amyloid plaques, neurofibrillary tangles, and other types of brain pathology.”
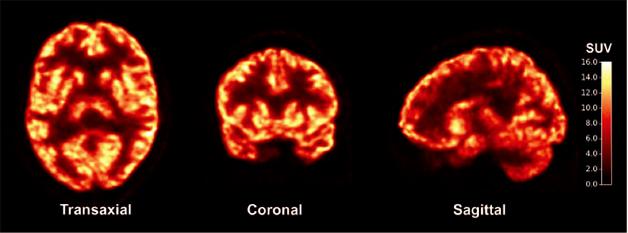
Synaptic signal. [11C]UCB-J binds synapses and lights up in a PET scan of a healthy subject. [Science Translational Medicine/AAAS.]
Until now, researchers could only measure synaptic density in postmortem tissue by using antibodies to detect proteins such as synaptophysin (Buckley and Kelly, 1985). A molecule that binds synaptophysin might prove useful as a PET ligand but scientists have yet to find one. However, levetiracetam—an anti-epileptic drug—targets a similarly widespread protein called synaptic vesicle glycoprotein 2A (SV2A). Previous studies have reported that every synaptic vesicle in the brain carries two to five copies of this protein, said Finnema. UCB, the Brussels-based company that developed levetiracetam and provided some of the funding for this study, derived several PET tracer candidates based on this drug. A couple have been tested in animals and humans, but no reports on human brain imaging had been published (Estrada et al., 2016; Bretin et al., 2015). Carson, who directs the Yale PET Center, which specializes in developing and using new PET ligands, chose one of the derivatives, [11C]UCB-J, to test in people.
Using non-human primates first, the researchers found that PET scans lit up everywhere there was a functioning synapse (Nabulsi et al., 2016). To confirm that the [11C]UCB-J PET signal measured synapse density, Finnema and colleagues compared an in vivo PET scan of a baboon with postmortem analysis of its brain tissue The PET signal in 11 areas of gray matter closely matched the density of SV2A protein as measured in western blots. There was very little signal in the centrum semiovale, a white matter area, in either the PET scan or the westerns. Western blot analyses also revealed the densities of SV2A and synaptophysin tracked closely. Using high-powered confocal microscopy on a section of the somatosensory cortex, the authors found that both proteins turned up in dendrites, but not neuronal cell bodies. Combined, the data suggested that SV2A made an effective surrogate for synaptophysin. [11C]UCB-J bound SV2A with high affinity and at low nanomolar concentrations.
The Human Test
Would the tracer prove as effective in people? To find out, the researchers injected [11C]UCB-J intravenously into five healthy subjects, average age 37, and conducted PET scans using a High Resolution Research Tomograph, which resolves signals down to about 3mm, about twice as good as the typical PET scanner. The compound was effectively taken up by the brain, peaking in gray matter regions about 15 minutes after injection, meaning the entire process from injection to scan could conceivably take as little as an hour, Finnema said. Gray matter bound most of the tracer, contrasting starkly with the low uptake in white matter regions such as the centrum semiovale. Using the latter as a reference region gave peak standard uptake value ratios (SUVRs) of seven to 11 in cortical regions (see image above). Studies in three more healthy subjects suggested UCB-J was indeed binding SV2A; when the researchers injected levetiracetam halfway through the imaging procedure, the signal diminished, indicating that the two compounds compete for the same binding site.
To test if the ligand could be used to detect synaptic loss, the researchers then turned to three patients, average age 52, with epilepsy. All had mesial temporal lobe sclerosis on one side of the brain, meaning neurons had died away where the seizures originated, near the hippocampus. Compared to the healthy side, the affected hippocampus of each patient bound 40 to 60 percent less [11C]UCB-J (see image below). That signal loss matched the atrophy visible in MRI.
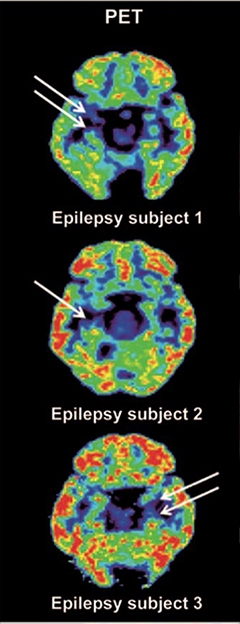
Asymmetrical signal.
Less [11C]UCB-J binding in the temporal lobe seizure onset areas in three epilepsy patients. [Science Translational Medicine/AAAS.]
According to Carson, the one big downside to the tracer is that it is labeled with C11, which has a half-life of only 20 minutes. This means [11C]UCB-J will be limited to centers with a nearby cyclotron for now. However, UCB-J contains three fluorine atoms, meaning it can, in principle, be labeled by F18, which has a half-life of almost two hours, said Carson. The C11 version is approved by the FDA for research purposes, but researchers will wait for F18-labeled compounds to seek approval for broader clinical use.
The authors are now working out which area of the brain would best serve as a reference region for generating SUVRs. It could be the centrum semiovale, which according to western blots should have no SV2A binding. However, the displacement PET studies with levetiracetam hinted at small amount of binding there.
“The authors have checked off the list of essential properties required of a useful PET radiopharmaceutical, and [11C]UCB-J has passed nearly all with flying colors,” wrote Chet Mathis, University of Pittsburgh, to Alzforum (see full comment below). The ligand could help determine how early in neurodegenerative diseases synaptic losses appear and how important assessment of synaptic losses will prove to be, he added. However, he cautioned that using the relatively small centrum semiovale as a reference region could be problematic in clinical PET scanners that have less than the relatively sharp 3mm resolution of the scanner used in this study.
“This looks like a promising tracer,” said Reisa Sperling, Brigham and Women’s Hospital, Boston. It would be important to see how it performs across the spectrum of early AD, to see at what stage synaptic density changes, she said. She also wondered what this tracer might detect before that loss, when synapses are hyperactive. Regardless, [11C]UCB-J might provide a specific way to track disease progress and tell whether drugs slow or reverse it. “So far we can’t regrow neurons, but maybe we can remodel and regrow synapses,” she told Alzforum. “I think the potential of being able to pick up a therapeutic effect before neurons are dead is important.”
Carson’s group is now scanning patients with AD and MCI, as well as older controls, to see how [11C]UCB-J binding compares to that seen in younger controls. Co-authors are also collaborating with other labs to image patients with schizophrenia, depression, Parkinson’s disease, traumatic brain injury, and other disorders with autopsy evidence of synapse damage. “If we can monitor synapse loss in living people, and see the time course and the effect of drugs, the possibility of impact on these diseases is dramatic,” Carson told Alzforum. He estimates that, given the interest he’s seen at conferences, the tracer will likely be used at other centers in the next 6 to 12 months. “We’re excited to add this into the armamentarium of imaging in AD. It could be an extremely important tool to help understand how amyloid and tau deposition interact with synaptic loss and lead to cognitive deficits.” Scientists led by Keith Johnson at MGH recently uncovered sites in the brain where tau and Aβ pathologies might interact to exacerbate the spread of disease (see Jul 2016 news).
Arthur Toga, University of Southern California, Los Angeles, pointed out that subjects can receive only so many radioactive tracers at a time, but argued in favor of as full a characterization of AD pathophysiology as possible. This tracer may enable scientists to better measure changes in synaptic density that relate to tissue atrophy changes seen using magnetic resonance imaging, he said.
Lennart Mucke, Gladstone Institute of Neurological Disease, San Francisco, California, agreed that the tracer could be influential. “Being able to non-invasively monitor the effects of neurodegenerative disorders and related therapeutics on synapse density could be a great advantage,” he wrote to Alzforum. He called the SV2A ligand promising, suggesting it could become a particularly meaningful and sensitive biomarker for Alzheimer’s disease. However, he agreed with the authors and other commentators that these results will have to be validated in larger numbers of people and in relevant patient populations.—Gwyneth Dickey Zakaib
References
Therapeutics Citations
News Citations
Paper Citations
- Buckley K, Kelly RB. Identification of a transmembrane glycoprotein specific for secretory vesicles of neural and endocrine cells. J Cell Biol. 1985 Apr;100(4):1284-94. PubMed.
- Estrada S, Lubberink M, Thibblin A, Sprycha M, Buchanan T, Mestdagh N, Kenda B, Mercier J, Provins L, Gillard M, Tytgat D, Antoni G. [(11)C]UCB-A, a novel PET tracer for synaptic vesicle protein 2A. Nucl Med Biol. 2016 Jun;43(6):325-32. Epub 2016 Mar 17 PubMed.
- Bretin F, Bahri MA, Bernard C, Warnock G, Aerts J, Mestdagh N, Buchanan T, Otoul C, Koestler F, Mievis F, Giacomelli F, Degueldre C, Hustinx R, Luxen A, Seret A, Plenevaux A, Salmon E. Biodistribution and Radiation Dosimetry for the Novel SV2A Radiotracer [(18)F]UCB-H: First-in-Human Study. Mol Imaging Biol. 2015 Aug;17(4):557-64. PubMed.
- Nabulsi NB, Mercier J, Holden D, Carré S, Najafzadeh S, Vandergeten MC, Lin SF, Deo A, Price N, Wood M, Lara-Jaime T, Montel F, Laruelle M, Carson RE, Hannestad J, Huang Y. Synthesis and Preclinical Evaluation of 11C-UCB-J as a PET Tracer for Imaging the Synaptic Vesicle Glycoprotein 2A in the Brain. J Nucl Med. 2016 May;57(5):777-84. Epub 2016 Feb 4 PubMed.
Further Reading
Papers
- Mercier J, Archen L, Bollu V, Carré S, Evrard Y, Jnoff E, Kenda B, Lallemand B, Michel P, Montel F, Moureau F, Price N, Quesnel Y, Sauvage X, Valade A, Provins L. Discovery of heterocyclic nonacetamide synaptic vesicle protein 2A (SV2A) ligands with single-digit nanomolar potency: opening avenues towards the first SV2A positron emission tomography (PET) ligands. ChemMedChem. 2014 Apr;9(4):693-8. Epub 2014 Jan 20 PubMed.
- Overk CR, Masliah E. Pathogenesis of synaptic degeneration in Alzheimer's disease and Lewy body disease. Biochem Pharmacol. 2014 Apr 15;88(4):508-16. Epub 2014 Jan 21 PubMed.
News
- Overactive Kinase May Drive Aβ to Depress Synapses
- Microglia Prune Synapses in a Subtype of Frontotemporal Dementia
- Paper Alert: Microglia Mediate Synaptic Loss in Early Alzheimer’s Disease
- PTEN Makes Aβ Depressingly Toxic
- Calcium Disruptions Wreak Synaptic Havoc
- Synaptic Genes Determine Brain Connectivity
Primary Papers
- Finnema SJ, Nabulsi NB, Eid T, Detyniecki K, Lin SF, Chen MK, Dhaher R, Matuskey D, Baum E, Holden D, Spencer DD, Mercier J, Hannestad J, Huang Y, Carson RE. Imaging synaptic density in the living human brain. Sci Transl Med. 2016 Jul 20;8(348):348ra96. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
PET Facility
This study by Finnema et al. with the new SV2A PET radioligand, [11C]UCB-J, a putative marker of synaptic density, could be an important advance for neurodegenerative disease studies. [11C]UCB-J is highly selective for the SV2A protein, has low nanomolar affinity for SV2A, has a relatively high Bmax value in the 100-400 nM range, has high brain uptake, is well-behaved pharmacokinetically, has a relatively high specific signal in cortical areas, and a relatively low non-specific signal in a reference region (centrum semiovale), and in in vivo displacement studies using levetiracetam it demonstrated rapid loss of cortical signal.
Correspondence of the staining and western blots of SV2A and synaptophysin (an established marker of synaptic density) in postmortem tissues were encouraging, but additional fully quantitative comparison studies would be helpful in making a solid case for UCB-J as a synaptic density marker equivalent to synaptophysin. The use of the relatively small centrum semiovale as a reference region is problematic in PET scanners not possessing the relatively high 3mm resolution of the HRRT scanner used. Additionally, the centrum semiovale showed a slightly perceptible decrease in signal following injection of displacement doses of levetiracetam, potentially indicating a small level of specific signal in that reference region. While use of the 11C radiolabel is expedient for research studies, the longer-lived 18F radiolabel is preferred for more widespread distribution of the radiotracer. The authors suggest that UCB-J could be radiolabeled with 18F, but this has not been demonstrated to date. Overall, [11C]UCB-J appears to be a very promising PET radiotracer to access regional brain synaptic densities in vivo.
Important questions it could help address include: How early in neurodegenerative disease processes do synaptic losses appear and how important will assessment of synaptic losses prove to be? It is widely assumed that regional FDG brain metabolic signal is primarily reflective of local synaptic activity. Would a more direct measure of regional synaptic densities be more sensitive to early dysfunction in synaptic processes than FDG? The answer to this and other questions appears to now be possible with [11C]UCB-J PET studies.
Synaptic loss is a key event in Alzheimer’s disease—so this imaging would help us better study timeline and triggers for AD and identify neuroprotective drugs. This ligand looks promising but needs more validation. For example, we still don’t know if it will convey different information from FDG-PET. If it works out, it could become a critical part of the gold standard of diagnosis and we could be rewriting our diagnostic criteria and disease timelines. But it will be at least three more years before this comes to market in a best case scenario for approval.
Brown University
The potential to obtain a reliable quantitative imaging measure of synaptic density would be a great advance in AD biomarker research. This report of 11C UCB-J, which binds to protein SV2A, may be a good first step toward this goal. Though the pathological validation was limited to a single baboon and the human sample involved imaging in only three patients with partial complex epilepsy, the preliminary data is encouraging that the tracer is binding to a meaningful synaptic marker. Much work needs to be done to develop this compound. A much larger validation study is needed, followed by testing in other disorders, including across the stages of AD. Further exploration is required to identify the optimum reference region. If the findings hold up, then labeling one of the fluorine atoms for widespread use would be essential. This type of tracer, once validated, could be a potential marker of AD risk, staging, and treatment outcome.
University of California, San Diego
This is potentially very interesting, and may be usable in a number of diseases in which synapse number is thought to be an important variable. It is not clear what is the sensitivity of this method; while differences were noted in temporal lobe epilepsy, this may be detected with other methods, too. In a similar vein, the amount of signal (specific to synapses) and background from the human images is not clear (I'm not very familiar with the levetiracetam method of displacement; how specific this is for synapses, etc.). It would be important to determine this. Obtaining quantitative data is important; correlating this to actual synapse density would be important; it seems this could be done in a baboon (EM analysis of a different regions after they are imaged in vivo).
Global Alzheimer's Platform Foundation
Advances in PET imaging, particularly development of radioligands binding to biologically important sites, has helped with major advances in clinical neuroscience research. This paper shows what appears to be the first radioligand to bind to sites at synapses in brain. If confirmed in other studies, this could provide a way to measure synaptic density in living humans and also to track changes in density regionally and with disease. These scans would complement other newly developed radioligands that allow imaging of amyloid plaques, neurofibrillary ranges, and other types of brain pathology.
This radioligand could be helpful in providing a pharmacodynamic marker of drug effects to enhance synaptic density. As such, it could help select among various drug candidates for clinical testing and for setting doses to be tested for clinical efficacy.
Overall, a very positive development if confirmed.
Make a Comment
To make a comment you must login or register.