News From the PET Front: Early Amyloid Networks and Tau Mystery
Quick Links
The advent of PET to image Alzheimer’s pathology in people has handed researchers new tools for staging disease during life, but PET also presents mysteries. At the first Advances in Alzheimer’s and Parkinson’s Therapies Focus Meeting (AAT-AD/PD) March 15–18 in Turin, Italy, Swedish researchers introduced a new method for analyzing amyloid accumulation in brain networks that could become an extremely early biomarker in people who have not yet reached the brain-wide cutoff for amyloid positivity. Other speakers debated an unexpected conundrum in tau PET, where older AD patients have less tau signal than younger ones. Do other pathologies fuel dementia in older people, or does the finding reflect a difference in how the brain handles tau with age? Meanwhile, other Swedish researchers showed the latest on an antibody-based PET ligand that detects oligomeric Aβ. The technique remains preclinical, but new data demonstrates that the ligand can detect a drop in Aβ after mice are treated with a BACE inhibitor. Beyond these three news flashes, AAT-AD/PD featured no major developments on PET, although longitudinal and multi-biomarker studies are ongoing.
- An amyloid network measure may help detect AD in people whose PET scan is below the threshold of positivity.
- Tau PET signals are lower in older AD patients than in younger.
- A PET tracer for oligomeric Aβ detects a treatment effect in mice.
Can the Pattern of Amyloid Deposition Predict Progression?
Previous work from researchers at Lund University, Sweden, indicated that Aβ42 levels in cerebrospinal fluid drop a few years before amyloid PET scans reach the brain-wide cutoff for positivity (Aug 2016 conference news). But PET studies have also shown that the brain accumulates patches of amyloid here and there before that brain-wide threshold is reached. Joana Pereira at Karolinska Institute in Stockholm wondered if this regional PET amyloid accumulation could become a biomarker in amyloid CSF–positive but PET-negative people. In collaboration with the Lund group, Pereira examined data from ADNI2 participants. In this cohort, 291 were CSF–/PET–, 81 CSF+/PET–, and 272 CSF+/PET+. She compared their amyloid PET to MRI scans to determine where in their brains amyloid accumulated, and used graph theory to analyze network relationships between 72 different regions.
In Turin, Pereira reported that amyloid burdens in particular brain regions correlated more closely with each other in CSF+ people than in controls. This correlation suggests a connection between these regions, Pereira noted. She saw this increased connectivity primarily for regions within the default mode network (DMN), while regions outside the DMN had less correlated amyloid loads than in controls.
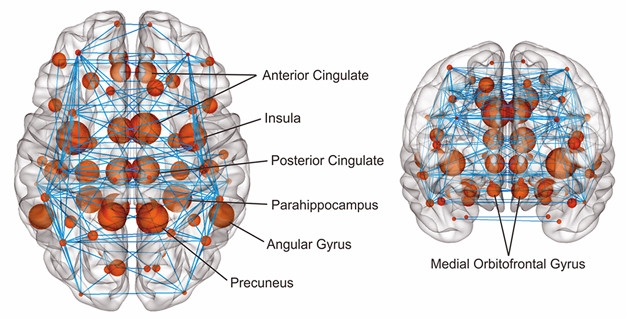
Amyloid Network. Red dots indicate regions with early amyloid accumulation, blue lines the connections between them. [Courtesy of Joana Pereira.]
Pereira also examined whether the correlations between regions were direct, or whether two given regions were connected through a third region. This measure is called network path length. Network paths within the DMN were shorter in CSF+/PET– people than in controls, she found, indicating a more direct connection between regions with high amyloid burden. This suggests amyloid is propagating through connected regions, Pereira said. In CSF+/PET+ people, network paths were shorter both within and outside the DMN.
Then Pereira analyzed a third measure. Clustering quantifies whether regions with correlated amyloid loads are close to each other in the brain. Here she saw no difference in CSF+ people. This, Pereira noted, implies that amyloid tends to accumulate in distant rather than neighboring brain regions; in other words, that the propagation happens via functional connections.
Of the three measures—connectivity, path length, and clustering—changes in network path length best discriminated between AD stages, Pereira said. She suggested this network measure could help pick out people on the path to AD and assess disease progression.
In addition, Pereira analyzed whole brain networks, and identified a subnetwork made up of regions that showed changes in connectivity and path length (see image above). This subnetwork expanded to include more regions in people with more amyloid pathology, again suggesting propagation along brain connections. It starts in medial regions, and spreads to lateral and frontal regions later in the disease. “This subnetwork could be more vulnerable to the effects of amyloid,” Pereira told Alzforum. The data were published in the January Cerebral Cortex (Pereira et al., 2018).
“This is very impressive data,” commented session co-chair Flavio Nobili of the University of Genoa, Italy. Many researchers asked technical questions. Some wanted to know how the amyloid network correlated with structural and functional changes. Pereira said she sees little change in brain volume at this early stage of the disease, when people are still 15 to 20 years away from developing symptoms. She is analyzing functional MRI data now. Previously, she found that the loss of structural connectivity correlated with neurodegeneration, but not amyloid, in the Swedish BioFINDER cohort (Pereira et al., 2017). Other work by the Swedish group has linked nascent amyloid accumulation in regions of the default mode network (DMN) with a loss of functional connectivity within that network, and between the DMN and other networks (Nov 2017 news).
Tau Pathology Looks Different in Middle-Aged and Elderly
Researchers have had less time to image tau than amyloid, and emerging findings still hold surprises. In Turin, Michael Weiner of the University of California, San Francisco, presented the latest tau PET data from ADNI, which by now has 240 baseline and 76 follow-up scans with AV1451. Tauopathy is believed to accelerate during symptomatic disease and for many years hopes were high that tau would be a straightforward progression marker. But surprisingly, in ADNI the tau PET signal was not higher in people with AD than in those with mild cognitive impairment. This finding appears to be age-dependent, Weiner noted. The average age of these ADNI participants was 79, whereas in a cohort of UCSF patients with an average age of 63, the tau signal did shoot up at the dementia stage (Maass et al., 2017). What is going on here? The answer is unknown, but will be important, Weiner said. He suggested the older group may have pathologies other than AD that contribute to their dementia.
Simon Lovestone at Oxford University added to this discussion when he presented preliminary findings from a pilot study of 24 people with mild AD, who agreed to undergo frequent extensive testing on a wide range of clinical and biomarker measures. The participants took clinical and cognitive batteries, gave samples of cerebrospinal fluid, blood, urine, and saliva, and underwent multiple brain-imaging techniques, including MRI, amyloid and tau PET, and EEG. They wore devices that recorded their gaits and navigation, and completed telephone assessments of verbal fluency, episodic memory, mood, and sleep patterns. Why gather so much data? There has long been dissatisfaction with the spotty, even misleading, results coming out of studies where measures that can be quite variable from day to day are taken but a few times, many months or a year apart (see Dec 2017 conference story). New approaches are trying to measure outcomes much more frequently or even continuously. The ultimate goal is to find biomarkers that could serve as outcome measures in secondary prevention trials, Lovestone said, and his current pilot study addressed whether it is feasible to collect this much information. The answer was a resounding yes, as the participants completed nearly all of the roughly 7,000 data points requested, Lovestone reported.
In his study, too, Lovestone was surprised to see that a participant’s tau burden was lower the older he or she was. He agreed with Weiner that dementia in older people could be due to factors other than AD pathogenesis; however, he also noted that older people appeared more susceptible to the toxic effects of tau, showing more cognitive impairment than a younger person with the same tau burden (Koychev et al., 2017). Thus, older people with high tau pathology simply may not show up in a group selected for mild dementia, he suggested.
Keith Johnson of Massachusetts General Hospital, Boston, agrees the most parsimonious explanation for the data is a survival effect, where older people with advanced tau pathology are more likely to die or progress to later disease stages, and thus are underrepresented in studies. He noted that amyloid accumulation follows a similar pattern as tau, with a robust age dependence that flattens out at old age and even dips slightly in the oldest old, supporting this idea. The age dependence of tau PET could complicate the interpretation of clinical trial data, and might require careful age balancing of treatment groups, Johnson wrote to Alzforum. Many current AD treatment trials include people ranging from age 50 to 80 in a given group.
For his part, William Jagust at the University of California, Berkeley, believes the age difference in tau PET scans may be saying something fundamental about the biology of tau. Jagust noted that several groups have found an inverse relationship between age and tau pathology. Research groups at UC Berkeley, UCSF, and in Sweden recently reported that younger AD patients have widespread neocortical tau, while older patients accumulate more focal tau in the hippocampus and medial temporal regions (Ossenkoppele et al., 2016; Schöll et al., 2017). Moreover, tau PET correlates more strongly with cognitive decline in younger than in older people (Aug 2017 conference news).
“[These findings] are part of a larger story about age and AD that has been around for decades,” Jagust wrote to Alzforum. He notes that AD tends to present slightly differently in younger and older people. People with early onset AD develop more cortical hypometabolism than do late-onset cases (Mielke et al., 1992; Kim et al., 2005). Because tau pathology correlates with metabolic decline, it is not surprising for PET to see more cortical tau in younger patients as well, Jagust said. He believes this reflects a distinction between typical late-onset AD and a hippocampal-sparing form of the disease that affects mostly younger people (Murray et al., 2011). “The phenomenology fits together and is quite consistent, but the biology is unexplained,” Jagust noted.

See Oligomers in Vivo? A new PET tracer detects a drop in this form of Aβ in mouse brain after treatment with BACE inhibitor NB-360. [Courtesy of Silvio Meier and Dag Sehlin.]
PET Ligand Detects Treatment Effect on Soluble Aβ
While tracers for fibrillary amyloid and tau paired helical filaments are being used more widely, scientists are working to develop new ligands for other players in AD pathogenesis. Researchers at Uppsala University, Sweden, previously introduced an antibody directed against oligomeric forms of Aβ that could detect soluble protofibrils in transgenic mice (Feb 2016 news; Sehlin et al., 2017). The tracer is based on the murine version of BioArctic’s BAN2401 antibody.
In Turin, Dag Sehlin at Uppsala showed new data. Silvio Meier in his group treated Tg-ArcSwe mice, which generate high levels of oligomeric peptide, with Novartis’ NB-360 BACE inhibitor from 10 to 13 months of age. At this age, the PET signal rises rapidly in untreated controls. In response to treatment, however, the PET signal dropped by about half, bringing it down to near baseline (see image above). The researchers confirmed this visual drop in soluble Aβ by also directly measuring the peptide in brain tissue of treated and untreated mice.
The findings suggest that the oligomeric tracer can detect a treatment effect, Sehlin noted. However, he said that the mice had few plaques at the tested age, leaving unclear whether the tracer could still detect a drop in soluble Aβ at more advanced stages of plaque pathology. In addition, technical hurdles remain before this tracer can be tested in people.—Madolyn Bowman Rogers
References
News Citations
- Refining Models of Amyloid Accumulation in Alzheimer’s Disease
- Daydreaming Network Serves as Ground Zero for Aβ Deposition
- Cognitive Testing Is Getting Faster and Better
- Data from DIAN Revise Familiar Biomarker Trajectories
- Can Antibody-Based PET Scans Pinpoint Aβ Oligomers in the Brain?
Therapeutics Citations
Research Models Citations
Paper Citations
- Pereira JB, Strandberg TO, Palmqvist S, Volpe G, van Westen D, Westman E, Hansson O, Alzheimer’s Disease Neuroimaging Initiative. Amyloid Network Topology Characterizes the Progression of Alzheimer's Disease During the Predementia Stages. Cereb Cortex. 2018 Jan 1;28(1):340-349. PubMed.
- Pereira JB, van Westen D, Stomrud E, Strandberg TO, Volpe G, Westman E, Hansson O. Abnormal Structural Brain Connectome in Individuals with Preclinical Alzheimer's Disease. Cereb Cortex. 2017 Oct 3;:1-12. PubMed.
- Maass A, Landau S, Baker SL, Horng A, Lockhart SN, La Joie R, Rabinovici GD, Jagust WJ, Alzheimer's Disease Neuroimaging Initiative. Comparison of multiple tau-PET measures as biomarkers in aging and Alzheimer's disease. Neuroimage. 2017 Aug 15;157:448-463. Epub 2017 Jun 3 PubMed.
- Koychev I, Gunn RN, Firouzian A, Lawson J, Zamboni G, Ridha B, Sahakian BJ, Rowe JB, Thomas A, Rochester L, Ffytche D, Howard R, Zetterberg H, MacKay C, Lovestone S, Deep and Frequent Phenotyping study team (. PET Tau and Amyloid-β Burden in Mild Alzheimer's Disease: Divergent Relationship with Age, Cognition, and Cerebrospinal Fluid Biomarkers. J Alzheimers Dis. 2017;60(1):283-293. PubMed.
- Ossenkoppele R, Schonhaut DR, Schöll M, Lockhart SN, Ayakta N, Baker SL, O'Neil JP, Janabi M, Lazaris A, Cantwell A, Vogel J, Santos M, Miller ZA, Bettcher BM, Vossel KA, Kramer JH, Gorno-Tempini ML, Miller BL, Jagust WJ, Rabinovici GD. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease. Brain. 2016 May;139(Pt 5):1551-67. Epub 2016 Mar 8 PubMed.
- Schöll M, Ossenkoppele R, Strandberg O, Palmqvist S, Swedish BioFINDER study, Jögi J, Ohlsson T, Smith R, Hansson O. Distinct 18F-AV-1451 tau PET retention patterns in early- and late-onset Alzheimer's disease. Brain. 2017 Sep 1;140(9):2286-2294. PubMed.
- Mielke R, Herholz K, Grond M, Kessler J, Heiss WD. Differences of regional cerebral glucose metabolism between presenile and senile dementia of Alzheimer type. Neurobiol Aging. 1992 Jan-Feb;13(1):93-8. PubMed.
- Kim EJ, Cho SS, Jeong Y, Park KC, Kang SJ, Kang E, Kim SE, Lee KH, Na DL. Glucose metabolism in early onset versus late onset Alzheimer's disease: an SPM analysis of 120 patients. Brain. 2005 Aug;128(Pt 8):1790-801. PubMed.
- Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW. Neuropathologically defined subtypes of Alzheimer's disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 2011 Sep;10(9):785-96. PubMed.
- Sehlin D, Fang XT, Meier SR, Jansson M, Syvänen S. Pharmacokinetics, biodistribution and brain retention of a bispecific antibody-based PET radioligand for imaging of amyloid-β. Sci Rep. 2017 Dec 8;7(1):17254. PubMed.
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.