Deadly Delivery: Microglia May Traffic Tau Via Exosomes
Quick Links
Researchers have known for some time that the tau protein wears walking shoes; it can pass between neurons and march across interconnected regions of the brain. Microglial journeymen may facilitate these interneuronal excursions, according to a study published in Nature Neuroscience on October 5. Researchers led by Tsuneya Ikezu at Boston University School of Medicine reported that microglia gobbled up tau from neurons, packaged it into tiny vesicles called exosomes, and spat it out again. Nearby neurons then ingested the cargo. Ridding the mouse brain of microglia or blocking the production of exosomes stymied the spread of tau from the entorhinal cortex to its next-door neighbor, the hippocampus. While other modes of tau transportation may also operate in the brain, the researchers propose that targeting this exosomal intermediary could slow the spread of toxic tau in Alzheimer’s disease. Alzforum first reported on this work from the 2014 Society for Neuroscience annual meeting (see Dec 2014 conference news).
“The findings are quite counterintuitive,” commented Steven Paul of Weill Cornell Medical College, New York, who was not involved in the study. “These results suggest that microglia are the bad actors, as they may be involved in the transmission of tau, rather than the clearance of it.”
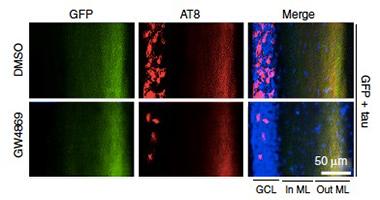
Delivery Derailed.
Phosphorylated tau (red) fails to travel to the granule cell layer in mice treated with an inhibitor of exosome synthesis (bottom panel). Each panel shows (from left to right) the granule cell layer, and the inner and outer molecular layers of the dentate gyrus. [Courtesy of Asai et al., Nature Neuroscience 2015.]
Mounting evidence suggests that aggregated forms of tau spread from one neuron to the next, where they corrupt normal forms of the protein (see June 2009 news; Iba et al., 2013). Tau aggregates first appear in the entorhinal cortex in the early stages of AD, and then spread to the hippocampus, where they are thought to damage neurons and cause memory problems (see Braak and Braak, 1991). By using mice in which tau expression is mostly restricted to the EC, researchers have reported that tau can spread across neuronal circuits that connect these two regions (see Feb 2012 news and Apr 2015 news). However, the way tau moves from one neuron to the next is unclear. Furthermore, tau sometimes travels off-road, wandering between neurons that are not connected by synapses.
Exosomes are one vehicle that could theoretically facilitate the transfer of tau along well-worn circuits as well as less-traveled paths. These tiny vesicular packages are released from cells and contain intracellular components. Researchers have detected elevated levels of tau-filled exosomes in the cerebrospinal fluid and blood of people with AD, which suggests that these tau packages are released from cells in the brain (see Saman et al., 2011; Fiandaca et al., 2014; and Aug 2014 conference news). While many types of cells, including neurons, can produce exosomes, microglia are the most adept at pumping them out. As the brain’s immune cells, microglia gobble up extracellular material and then either digest it or spit it back out via exosomes.
First author Hirohide Asai and colleagues wanted to test whether microglial exosomes facilitate the movement of tau between neurons. They generated a viral model of rapid spread by injecting mouse EC with adeno-associated viruses (AAV) that express green fluorescent protein (GFP) and the P301L variant of human tau, which causes frontotemporal dementia. An array of antibodies specific for phosphorylated tau (AT8), human tau (HT7), and tau oligomers (T22) detected the protein, but not GFP, in dentate granule cells of the hippocampus 28 days after infection.
While the bulk of the cells containing tau were neurons, nearly 20 percent were microglia, as detected by staining with the P2ry12 antibody. The researchers found that 80 percent of the tau-bearing cells in the granule cell layer also stained positive for activated caspase 3, a marker of apoptosis. Programmed death would make the cells prime targets for microglial nibbling, the researchers proposed. Corroborating the findings from their AAV model, the researchers used both immunofluorescence and electron microscopy to look for phospho-tau in microglia from PS19 mice, which express human P301L. The microglia harbored electron-dense material that appeared to be protein aggregates.
To determine whether microglia played a role in the transfer of tau from the EC to the DG, the researchers depleted them in two different ways. In one, they infused clodronate liposome directly into the brain. This poison kills phagocytic cells, including microglia, after they engulf it. Alternatively, they fed the mice with chow containing PLX3397, an inhibitor of colony stimulating factor 1. Microglia rely upon CSF1 for survival and thus fade away when the factor is blocked. These methods reduced the number of microglia in the brain—by 70 and 86 percent, respectively. Strikingly, when mice were given either treatment for a month following EC infection with AAV-tau, the amount of phospho-tau found in the granule cell layer also dropped by 72 and 86 percent. The researchers observed substantial reductions in phospho-tau in the EC and DG of PS19 mice treated with PLX3397 as well.
To determine if tau spewed by microglia affected neuronal function, the researchers took field recordings of DG neurons in hippocampal slices after stimulating them with an electrical current. DG neurons from mice previously infected with AAV-tau had substantially lower spike amplitudes than mice infected with AAV-GFP, suggesting that tau reduced the excitability of neurons in the DG. However, no drop in excitability occurred in AAV-tau-infected mice that had been depleted of microglia.
Could microglia pass tau around via exosomes? To find out, the researchers first checked whether primary microglial cultures could take up aggregated tau and pump it out in exosomes. Western blots of cell extracts revealed that microglia readily took up pre-aggregated human tau via phagocytosis, whereas neurons and astrocytes did so poorly. Only exosomes secreted from microglia contained tau and those only when the microglia were treated with lipopolysaccharide and ATP, which together boost exosome packaging and secretion. The researchers next injected those tau exosomes directly into the outer layer of the DG in mice. Immunofluorescence then detected both the exosomes and hTau within cells deeper in the granule cell layer of the gyrus. In contrast, when “naked” hTau protein was injected into the outer layer, none of the protein ended up in the granule cells. The results indicated that microglial-derived exosomes could transfer tau between neurons.
Would blocking the production of exosomes by microglia thwart the spread of tau? The researchers tested this by treating microglial cultures with GW4869, an inhibitor of neutral sphingomyelinase-2 (nSMase2). The enzyme is required for the synthesis of ceramide, an essential lipid component of exosomes. After confirming that the inhibitor blocked exosome production in microglial cultures, the researchers injected it once daily for a month into the abdomens of mice that had been infected with AAV-tau in the EC. GW4869 reduced the levels of phospho-tau in the dentate gyrus granule cell layer by 75 percent (see image above). Fourteen-week-old PS19 mice treated with the inhibitor also had less phospho-tau in that layer than untreated controls.
“The evidence that reducing microglia or their exosomes can impede the progression of tauopathy is tantalizing, and supports the concept that microglia are more important in tau pathology than had been previously recognized,” commented Lary Walker of Emory University in Atlanta. He cautioned, however, that long-term depletion of microglia or inhibition of exosomes would likely lead to side effects, given their importance in brain function.
Marc Diamond of the University of Texas Southwestern Medical Center in Dallas found the study intriguing, but noted that the findings did not link microglia or exosomes to true propagation of tauopathy. The experiments only examined movement of tau protein from one region to another, not the corruption of normally folded tau. He also noted that for several of the key experiments, the authors only looked at phospho-tau using the AT8 antibody, rather than aggregated forms of tau that would facilitate propagation. The numbers of animals used in many experiments were also exceedingly small, he added. “These provocative findings clearly deserve further study,” he said.
Efforts are underway to rid the brain of tau using antibodies, some of which may rely upon microglia to take up the immune complexes and degrade them. A recent study from Paul’s lab hinted that microglia facilitate antibody-mediated clearance of tau, though other antibodies reportedly do their business without the aid of these cells (see Jun 2015 news). How can researchers reconcile these two reported roles of microglia—that of tau destroyer versus that of tau transmitter? Ikezu said that perhaps as metabolism changes in the aging brain, microglia may become overwhelmed and switch from degradation mode into exosome mode. Alternatively, large amounts of aggregated proteins that build up with age (or are present in the AAV-tau mice) might exceed the capacity of the degradative protein machinery. The researchers have yet to precisely determine which forms of tau are packaged in exosomes versus taken up via antibodies or other routes.
Rakez Kayed of the University of Texas in Galveston found the study impressive. He added that the microglia/exosome pathway likely represents only one of several routes of tau transport in the brain. Kayed speculated that some species of tau may pass predominantly between neurons at synapses, and others, perhaps larger ones, via exosomes. “We need to determine which species of tau are most efficient at spreading between neurons, and develop ways to block that spread,” he said.—Jessica Shugart
References
News Citations
- Exosomes: Purveyors of Neurodegenerative Disease?
- Traveling Tau—A New Paradigm for Tau- and Other Proteinopathies?
- Mice Tell Tale of Tau Transmission, Alzheimer’s Progression
- Propagation Blues? Reporter Expression Clouds Reports of Traveling Tau, Aβ
- Exosomes Stand Out as Potential Blood Biomarkers
- Antibodies Boost Microglial Appetite for Tau
Research Models Citations
Paper Citations
- Iba M, Guo JL, McBride JD, Zhang B, Trojanowski JQ, Lee VM. Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer's-like tauopathy. J Neurosci. 2013 Jan 16;33(3):1024-37. PubMed. Correction.
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239-59. PubMed.
- Saman S, Kim W, Raya M, Visnick Y, Miro S, Jackson B, McKee AC, Alvarez VE, Lee NC, Hall GF. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid (CSF) in early Alzheimer's Disease. J Biol Chem. 2011 Nov 4; PubMed.
- Fiandaca MS, Kapogiannis D, Mapstone M, Boxer A, Eitan E, Schwartz JB, Abner EL, Petersen RC, Federoff HJ, Miller BL, Goetzl EJ. Identification of preclinical Alzheimer's disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimers Dement. 2014 Aug 14; PubMed.
Other Citations
Further Reading
No Available Further Reading
Primary Papers
- Asai H, Ikezu S, Tsunoda S, Medalla M, Luebke J, Haydar T, Wolozin B, Butovsky O, Kügler S, Ikezu T. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci. 2015 Nov;18(11):1584-93. Epub 2015 Oct 5 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Mayo Clinic Florida
Thank you for the encouraging comments and critiques on our article. Regarding the species of tau transmitting in this study, the propagated tau was immunoreactive to oligomer-specific antibody (T22), and we saw more AT8+ tau at a later stage, which is commonly seen in the brain of advanced AD stage, although these were thioflavin-S negative. We agree that further studies are necessary.
One interesting topic raised by these comments is whether neurofibrillary tangle formation is necessary for neurodegeneration. There is a clear disconnect between neuronal cell loss and neurofibrillary tangle formation as reported in the rTg4510 mouse model (Santacruz et al., 2005; Spires et al., 2006). This demonstrates that neuronal cell loss precedes NFT formation, thus NFT is not necessarily toxic to neurons.
Indeed, others reported that NFT-bearing neurons maintain excitability as determined by whole-cell path clamp recording of cortical neurons of rTg4510 mice (Crimins et al., 2011). On the other hand, tau oligomer is neurotoxic and can propagate in wild-type mouse brain (Lasagna-Reeves et al., 2010; Lasagna-Reeves et al., 2012). Thus, non-fibril tau can propagate and is toxic to neurons, demonstrating the biological importance of non-fibril tau in pathobiology of AD.
References:
Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe KH. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005 Jul 15;309(5733):476-81. PubMed.
Spires TL, Orne JD, Santacruz K, Pitstick R, Carlson GA, Ashe KH, Hyman BT. Region-specific dissociation of neuronal loss and neurofibrillary pathology in a mouse model of tauopathy. Am J Pathol. 2006 May;168(5):1598-607. PubMed.
Crimins JL, Rocher AB, Peters A, Shultz P, Lewis J, Luebke JI. Homeostatic responses by surviving cortical pyramidal cells in neurodegenerative tauopathy. Acta Neuropathol. 2011 Nov;122(5):551-64. PubMed.
Lasagna-Reeves CA, Castillo-Carranza DL, Guerrero-Muoz MJ, Jackson GR, Kayed R. Preparation and characterization of neurotoxic tau oligomers. Biochemistry. 2010 Nov 30;49(47):10039-41. Epub 2010 Nov 8 PubMed.
Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, Guerrero-Munoz MJ, Kiritoshi T, Neugebauer V, Jackson GR, Kayed R. Alzheimer brain-derived tau oligomers propagate pathology from endogenous tau. Sci Rep. 2012;2:700. PubMed.
Make a Comment
To make a comment you must login or register.