Gene Networks That Control Astrocyte Diversity Linked to AD
Quick Links
Astrocytes, the so-called helper cells of the brain, permeate the entire central nervous system. How similar are these cells to one another? Not very, according to a new transcriptomic analysis. Researchers led by Baljit Khakh, University of California, Los Angeles, found 825 commonly expressed genes but 3,500 differentially expressed ones among mouse astrocytes throughout the brain. Dozens of the latter control morphology. Knocking down just two of these, FERMT2 and EZR, shrunk astrocytes, impaired synapses, and worsened memory. Other morphology-related genes are known risk genes for neurodegenerative diseases. In a mouse model of amyloidosis, astrocytes downregulated such genes and they were smaller than those from wild-type mice. The research, published November 3 in Science, offers molecular explanations for astrocyte shape and why it changes in neurodegenerative diseases.
- Astrocyte gene expression varies across the brain.
- Some differentially expressed genes control morphology.
- They include risk genes for AD and other neurodegenerative diseases.
“This study shows that astrocytes are heterogeneous both within and between CNS regions, displaying subtle molecular and morphological specificities that require potent and dedicated techniques to evidence them,” wrote Carole Escartin of CNRS, Université Paris-Saclay, France (full comment below). However, other researchers were concerned that the sequencing was underpowered and that too much emphasis was placed on cell morphology, a decades-old approach to studying astrocyte heterogeneity.
Scientists have long recognized that astrocytes can assume distinct morphological shapes. More recently, they have used single-cell RNA sequencing to identify different transcriptional states as well, including those that emerge in disease (Jul 2022 news; Jun 2022 news). However, nobody has directly compared astrocytes across the entire CNS, noted Khakh and colleagues.
To do so, first author Fumito Endo collected tissue from 13 brain and spinal cord regions of wild-type mice. Then he isolated astrocyte RNA by specifically tagging astrocytic ribosomes with hemagglutinin, then immunoprecipitating those complexes along with any mRNAs they are actively transcribing. He used RNA-Seq to capture gene expression patterns, then compared them with bulk tissue RNA-Seq to identify which transcripts were specific to astrocytes. Of 4,314 transcripts enriched in astrocytes, 825, including the known astrocyte markers APOE and Slc1a2, were expressed in cells from all areas. Many of these transcripts encode proteins involved in core astrocytic tasks, such as neurotransmitter homeostasis, cholesterol biosynthesis, and glucose metabolism. However, little is known about one-third of the genes.
Turning their attention to what differentiated astrocytes from one another, the scientists focused on the remaining 3,489 genes differentially expressed by brain region. These included genes involved in calcium flux across the cell membrane and G protein-coupled receptor signaling. The authors chalked up regional differences to the tissue microenvironment of each brain area. In keeping with this idea, subclusters of astrocytes expressed genes that respond to specific neurotransmitters, growth factors, and cytokines.
To delve deeper into astrocyte differences, the researchers used single-cell RNA-Seq from cortical, hippocampal, and striatal tissue. Of the 89,500 isolated cells, 8,900 were astrocytes. Their transcriptomes clustered into seven subgroups. The proportion of each subcluster varied by brain region (see image below).
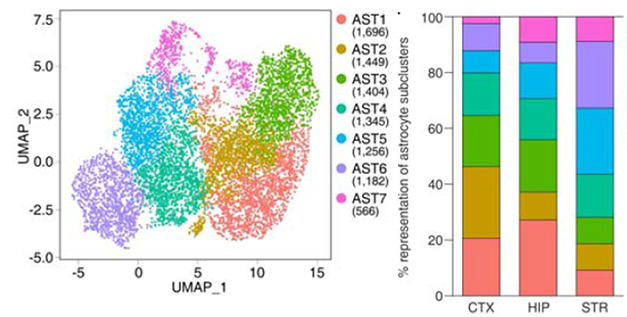
Regional Variations. Astrocyte transcriptomes from wild-type mice fell into seven clusters (left), which were present at different proportions in the cortex, hippocampus, and striatum (right). [Courtesy of Endo et al., Science, 2022.]
Endo found that some transcripts in these subclusters correlated with astrocyte morphologies, such as roundness, soma size, and number of cell branches. Using weighted gene co-expression analyses, he found that two gene networks controlled much of this morphology. These included the genes for Fermt2 and Ezrin, two proteins that interact with actin and likely mold the cytoskeleton.
To see how these genes affected the cells, the researchers knocked them down in adult wild-type mice, using CRISPR/Cas9 to suppress expression specifically in astrocytes. Two weeks later, expression of Fermt2 and Ezrin had fallen about 75 percent. Astrocytes shrank, hippocampal synapses shriveled, and the mice had difficulty in an object location test of memory. “This study further supports the idea that astrocyte morphology is key for their interactions with neurons,” wrote Escartin.
The researchers were struck that FERMT2 and other genes associated with morphology, such as APOE and CLU, are known risk genes for AD (Apr 2022 news; Oct 2020 news; Jan 2018 news). Curious if morphology-related gene expression changes in the context of amyloidosis, the scientists used scRNA-Seq to identify astrocyte transcriptomes from cortical tissue from 10-month-old wild-type and APP/PS1 mice. The latter had extensive amyloid plaques and gliosis. Of the 45,000 isolated cells, 3,800 were astrocytes. Compared to wild-types, the APP/PS1 astrocytes downregulated expression of homeostasis and morphology genes, and they were slightly smaller than normal cells.
What about astrocytes in other neurodegenerative diseases? These findings mirror prior studies in a mouse model of Huntington’s disease where Khakh found that those astrocytes were smaller than in wild-type mice (Octeau et al., 2018; Yu et al., 2020). Now, by searching through a previously published dataset of astrocyte transcriptomes from the same HD mouse model and another brain transcriptome dataset from people who had had HD, Endo found that HD astrocytes also downregulated morphology-related genes (Lee et al., 2020).
To look more broadly, Endo surveyed Phenopedia, a database of genetic variants that have been reported to associate with one or more of 3,400 diseases. Indeed, a few morphology-related genes, including FERMT2 and Slc1a2, appeared to be linked to AD, amyotrophic lateral sclerosis, and multiple sclerosis. All told, the authors concluded that loss of complex astrocyte morphology and homeostasis may be common in neurodegenerative diseases.
However, others were cautious about interpreting the RNA-Seq results. Some believe the study was underpowered because too few astrocytes were analyzed per brain region from each mouse and there were too few cells per subtype.
Others wondered if the sample may have been contaminated, because the microglia genes Trem2, Tyrobp, and C1qa, and the oligodendrocyte gene Mbp appeared as top differentially expressed genes in APP/PS1 astrocytes, despite not being considered astrocyte genes. Researchers recently reported neuronal RNA contaminating glial transcriptomes (Caglayan et al., 2022). This “ambient” RNA skewed cell annotations and masked the presence of rare glia subtypes. “Ambient RNA is a common problem in single-cell and single-nucleus sequencing and needs to be accounted for before downstream analysis,” Samuel Marsh, Boston Children’s Hospital, told Alzforum.
Khakh does not believe ambient RNA is a problem in the current study because he also detected the microglial and oligodendrocyte genes in RNA isolated from wild-type astrocytes by ribosomal immunoprecipitation. Instead, he suspects that these genes are expressed at low levels in astrocytes and that perhaps Aβ evokes their upregulation, though he did not test that idea directly.—Chelsea Weidman Burke
References
News Citations
- Don’t Name It: Glial States Confound Easy Labels
- Astrocyte Reactivity: Opposing States Emerge
- Paper Alert: Massive GWAS Meta-Analysis Published
- Largest Alzheimer GWAS in African Americans Finds New Variants
- Scanning Top 20 GWAS Hits for Amyloidosis: Slim Pickings
Research Models Citations
Paper Citations
- Octeau JC, Chai H, Jiang R, Bonanno SL, Martin KC, Khakh BS. An Optical Neuron-Astrocyte Proximity Assay at Synaptic Distance Scales. Neuron. 2018 Apr 4;98(1):49-66.e9. PubMed.
- Yu X, Nagai J, Marti-Solano M, Soto JS, Coppola G, Babu MM, Khakh BS. Context-Specific Striatal Astrocyte Molecular Responses Are Phenotypically Exploitable. Neuron. 2020 Dec 23;108(6):1146-1162.e10. Epub 2020 Oct 20 PubMed.
- Lee H, Fenster RJ, Pineda SS, Gibbs WS, Mohammadi S, Davila-Velderrain J, Garcia FJ, Therrien M, Novis HS, Gao F, Wilkinson H, Vogt T, Kellis M, LaVoie MJ, Heiman M. Cell Type-Specific Transcriptomics Reveals that Mutant Huntingtin Leads to Mitochondrial RNA Release and Neuronal Innate Immune Activation. Neuron. 2020 Sep 9;107(5):891-908.e8. Epub 2020 Jul 17 PubMed.
- Caglayan E, Liu Y, Konopka G. Neuronal ambient RNA contamination causes misinterpreted and masked cell types in brain single-nuclei datasets. Neuron. 2022 Sep 27; PubMed.
External Citations
Further Reading
No Available Further Reading
Primary Papers
- Endo F, Kasai A, Soto JS, Yu X, Qu Z, Hashimoto H, Gradinaru V, Kawaguchi R, Khakh BS. Molecular basis of astrocyte diversity and morphology across the CNS in health and disease. Science, November 4, 2022 Science
- Baldwin KT. Molecular diversity of astrocytes. Science, November 3, 2022 Science
Annotate
To make an annotation you must Login or Register.

Comments
UMR9199 Neurodegenerative Disease lab
This article by the Khakh lab characterizes the molecular and morphological features of astrocytes in 13 brain regions of the CNS, extending largely their previous study (Chai et al., 2017), where hippocampal and striatal astrocytes were thoroughly compared, including at the functional level.
The data generated will be a valuable resource for researchers studying astrocytes, providing extensive transcriptomics datasets as well as morphological parameters.
Surprisingly, Endo et al. show that the density of astrocytes across these 13 brain regions varies only by a factor of 2 and does not correlate with neuronal density. They also show variations of several metrics of astrocyte morphology, both within and between CNS regions. In fact, the most morphologically distinct astrocytes are found in the cerebellum, but this is probably due to the atypical morphology of Bergman glia, a specialized type of cerebellar glia.
Using advanced correlation analyses, Endo et al. identify modules of genes positively correlated to morphological features of astrocytes, which are mainly downregulated in neurodegenerative (ND) and psychiatric diseases. Indeed, astrocytes do display reduced territory in ND, as shown previously in a mouse model of AD (Olabarria et al., 2010) and replicated here in a different mouse model.
Then, they performed astrocyte-specific CRISPR knockdown of two genes encoding established cytoskeletal proteins (Fermt2 and Ezr) belonging to gene modules correlated with astrocyte morphological features. They show that this reduces astrocyte territory by 20 percent, altering memory, c-fos induction in neurons, and synapse number in the hippocampus.
Intriguingly, using scRNA-Seq, Endo et al. report the existence of seven sub-clusters of astrocytes in the cortex, striatum, and hippocampus, whose proportion differs slightly among these three regions and also in AD mice. It would now be interesting—but also very difficult, due to the lack of unique gene markers to identify these sub-clusters—to see whether specific sub-clusters are more prone to morphological alterations in disease.
This study further supports the idea that astrocyte morphology is key for their interactions with neurons (Pannasch et al., 2014; Stogsdill et al., 2017). It also shows that astrocytes are heterogeneous both within and between CNS regions, displaying subtle molecular and morphological specificities that require potent and dedicated techniques to evidence them. Future functional studies based on these rich datasets should provide insight into how astrocytes engage in region-specific interactions with neurons, through their finely controlled morphology.
References:
Chai H, Diaz-Castro B, Shigetomi E, Monte E, Octeau JC, Yu X, Cohn W, Rajendran PS, Vondriska TM, Whitelegge JP, Coppola G, Khakh BS. Neural Circuit-Specialized Astrocytes: Transcriptomic, Proteomic, Morphological, and Functional Evidence. Neuron. 2017 Aug 2;95(3):531-549.e9. Epub 2017 Jul 14 PubMed.
Olabarria M, Noristani HN, Verkhratsky A, Rodríguez JJ. Concomitant astroglial atrophy and astrogliosis in a triple transgenic animal model of Alzheimer's disease. Glia. 2010 May;58(7):831-8. PubMed.
Pannasch U, Freche D, Dallérac G, Ghézali G, Escartin C, Ezan P, Cohen-Salmon M, Benchenane K, Abudara V, Dufour A, Lübke JH, Déglon N, Knott G, Holcman D, Rouach N. Connexin 30 sets synaptic strength by controlling astroglial synapse invasion. Nat Neurosci. 2014 Apr;17(4):549-58. Epub 2014 Mar 2 PubMed.
Stogsdill JA, Ramirez J, Liu D, Kim YH, Baldwin KT, Enustun E, Ejikeme T, Ji RR, Eroglu C. Astrocytic neuroligins control astrocyte morphogenesis and synaptogenesis. Nature. 2017 Nov 8;551(7679):192-197. PubMed.
Make a Comment
To make a comment you must login or register.