Reverse Electron Flow in Microglia Linked to Neuroinflammation
Quick Links
Brain lesions beset with smoldering inflammation, and myeloid cells around their edges, define multiple sclerosis. This might be due, in part, to the flipping of electron flow within microglia, propose Luca Peruzzotti-Jametti, Stefano Pluchino, and colleagues at the University of Cambridge, U.K. In the March 13 Nature, they reported that in a mouse model of MS, disease-associated microglia seem to undergo reverse electron transport (RET), where mitochondria make reactive oxygen species (ROS) rather than ATP. Inhibiting RET lessened oxidative damage and neurodegeneration in spinal cords and shifted microglia back to homeostasis.
- Mice with MS-like brain inflammation flip electron flow in microglial mitochondria.
- These disease-associated microglia likely cause oxidative damage.
- Blocking reversed electron flow eased oxidative stress, neurodegeneration.
“This adds a new mechanistic layer to microglial biology in MS,” Costantino Iadecola of Weill Cornell Medical College, New York, told Alzforum. Russ Swerdlow, University of Kansas, Kansas City, called the observations in this study pertinent to AD (see comment below).
In MS, inflammation eats away the myelin sheath within the brain and spinal cord, creating patches of scarring on nerves. Microglia surround these lesions, spewing neurotoxic cytokines and ROS, and preventing remyelination (Zrzavy et al., 2017).
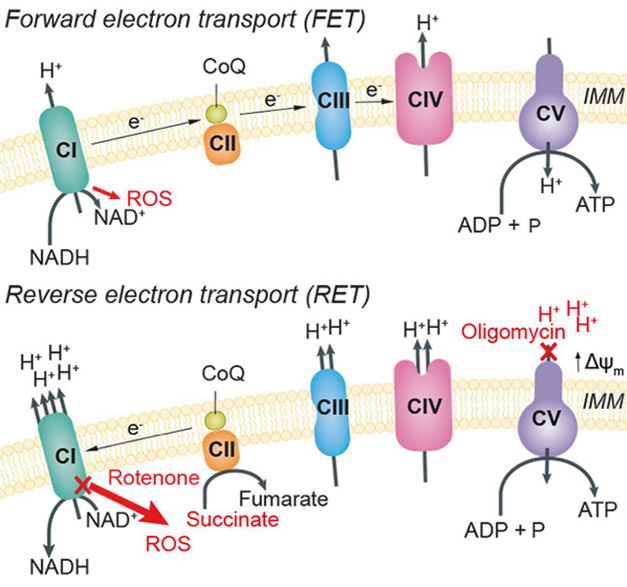
Flipping The Flow. In the inner mitochondrial membrane (IMM), electrons normally flow from complex I to IV (top, FET). When protons build up outside the membrane, or succinate inside, electrons switch and go from complex II back to complex I, creating excess ROS (bottom, RET). [Courtesy of Peruzzotti-Jametti et al., Nature, 2024.]
In cell culture, macrophages make ROS when stirred up by lipopolysaccharide (LPS). These cells switch from forward electron transport along the respiratory chain, i.e., from making ATP, to RET by upregulating mitochondrial complex I (image above; Mills et al., 2016). Might RET fuel microglial activation in MS?
To find out, co-first authors Peruzzotti-Jametti, Cory Willis, and Grzegorz Krzak used single-cell RNA-Seq and mass spectrometry to analyze microglia isolated from mice with experimental autoimmune encephalomyelitis (EAE), a model for MS-like brain inflammation, after three (acute) or 50 (chronic) days of illness.
The cells fell into 13 distinct clusters, of which two represented homeostatic cells and six were disease-associated microglia (DAM). The latter upregulated Spp1, Cst7, and ApoE, all DAM markers in Alzheimer’s disease (Oct 2020 news; Mar 2021 news). When Peruzzotti-Jametti analyzed two previously published single nuclei RNA-Seq datasets of human microglia isolated from people with progressive MS, he found a cluster similar to the EAE DAMs (Schirmer et al., 2019; Absinta et al., 2021).
In wild-type mice, almost all microglia were homeostatic. In acute EAE mice, fewer than 1 percent were; 46 percent were DAMs. In chronic EAE, one-third of microglia were homeostatic, 48 percent were DAMs. To the authors' minds, this implies that DAMs flare in the beginning of the disease course and stay steady, while homeostatic microglia die off early on, then are partially restored.
Spp1-positive DAMs flooded spinal cord tissue slices from EAE mice. They also surrounded lesions in postmortem brain tissue from people who had had progressive MS (image below).
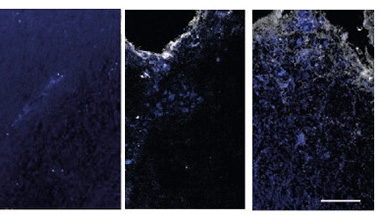
DAM Deluge. Wild-type mice (left) had no microglia expressing Spp1 (white), nor the mitochondrial complex I subunit NDUFS4 (blue) in their spinal cords. Acute EAE mice had some (middle); chronic EAE mice the most (right). [Courtesy of Peruzzotti-Jametti et al., Nature, 2024.]
The genes upregulated in EAE DAMs related to glycolysis and mitochondrial complex I, an expression pattern indicative of RET. Likewise, while acute EAE microglia contained high levels of ROS-scavenging molecules, antioxidant reaction byproducts, and RET-limiting molecules, chronic EAE microglia had less of these, along with a dearth of ATP, suggesting they made more ROS via RET.
Bingwei Lu of Stanford University was not completely convinced that RET was up in DAMs, because the authors showed only indirect evidence. “Still, they make a strong case for the involvement of RET in MS,” Lu told Alzforum.
He believes the case is strong because blocking RET in EAE mice eased pathology. The authors injected mice intraperitoneally with metformin, a diabetes drug that doubles as a mitochondrial complex I inhibitor, and the complex II inhibitor dimethyl malonate. A month later, these mice had less oxidative stress and neurodegeneration in their spinal cord tissue than did untreated mice. They also had 1.4 times more homeostatic microglia and 2.5-fold fewer DAMs. EAE mice with a mutation in the complex I gene had milder disease, with fewer DAMs and ROS, than EAE mice with a normal complex 1 gene.
All told, the authors believe mitochondrial RET in microglia might be a therapeutic target in MS and, possibly, other neurological diseases. “We think this mechanism is ubiquitous in chronically stimulated microglia,” Peruzzotti-Jametti told Alzforum.
Metformin is being trialed in multiple sclerosis, Alzheimer's, Parkinson's, and other neurologic diseases.
For his part, Lu previously reported similar RET activation in iPSC-derived neurons from AD patients and neurons of a fruit fly model of AD. Inhibiting RET also minimized disease phenotypes of the flies (Rimal et al., 2023; reviewed by Chavada and Lu, 2023). Another recent preprint described faulty microglial metabolism in AD, partially driven by mitochondria that were disabled by TREM1 (Mar 2024 news).
“Mitochondrial dysfunction is a major driver of aging, so I think RET could be pathogenic in any age-related neurodegenerative disease,” Lu told Alzforum. —Chelsea Weidman Burke
References
News Citations
- Single-nucleus RNA Sequencing Misses Activation of Human Microglia
- WAM! New Microglial Subtype Mops Up Moribund Myelin
- TREM1 Muddles Myeloid Cell Metabolism and Memory in Old Mice
Therapeutics Citations
Paper Citations
- Zrzavy T, Hametner S, Wimmer I, Butovsky O, Weiner HL, Lassmann H. Loss of 'homeostatic' microglia and patterns of their activation in active multiple sclerosis. Brain. 2017 Jul 1;140(7):1900-1913. PubMed.
- Mills EL, Kelly B, Logan A, Costa AS, Varma M, Bryant CE, Tourlomousis P, Däbritz JH, Gottlieb E, Latorre I, Corr SC, McManus G, Ryan D, Jacobs HT, Szibor M, Xavier RJ, Braun T, Frezza C, Murphy MP, O'Neill LA. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell. 2016 Oct 6;167(2):457-470.e13. Epub 2016 Sep 22 PubMed.
- Schirmer L, Velmeshev D, Holmqvist S, Kaufmann M, Werneburg S, Jung D, Vistnes S, Stockley JH, Young A, Steindel M, Tung B, Goyal N, Bhaduri A, Mayer S, Engler JB, Bayraktar OA, Franklin RJ, Haeussler M, Reynolds R, Schafer DP, Friese MA, Shiow LR, Kriegstein AR, Rowitch DH. Neuronal vulnerability and multilineage diversity in multiple sclerosis. Nature. 2019 Sep;573(7772):75-82. Epub 2019 Jul 17 PubMed.
- Absinta M, Maric D, Gharagozloo M, Garton T, Smith MD, Jin J, Fitzgerald KC, Song A, Liu P, Lin JP, Wu T, Johnson KR, McGavern DB, Schafer DP, Calabresi PA, Reich DS. A lymphocyte-microglia-astrocyte axis in chronic active multiple sclerosis. Nature. 2021 Sep;597(7878):709-714. Epub 2021 Sep 8 PubMed.
- Rimal S, Tantray I, Li Y, Pal Khaket T, Li Y, Bhurtel S, Li W, Zeng C, Lu B. Reverse electron transfer is activated during aging and contributes to aging and age-related disease. EMBO Rep. 2023 Apr 5;24(4):e55548. Epub 2023 Feb 16 PubMed.
- Chavda V, Lu B. Reverse Electron Transport at Mitochondrial Complex I in Ischemic Stroke, Aging, and Age-Related Diseases. Antioxidants (Basel). 2023 Apr 6;12(4) PubMed.
External Citations
Further Reading
No Available Further Reading
Primary Papers
- Peruzzotti-Jametti L, Willis CM, Krzak G, Hamel R, Pirvan L, Ionescu RB, Reisz JA, Prag HA, Garcia-Segura ME, Wu V, Xiang Y, Barlas B, Casey AM, van den Bosch AM, Nicaise AM, Roth L, Bates GR, Huang H, Prasad P, Vincent AE, Frezza C, Viscomi C, Balmus G, Takats Z, Marioni JC, D'Alessandro A, Murphy MP, Mohorianu I, Pluchino S. Mitochondrial complex I activity in microglia sustains neuroinflammation. Nature. 2024 Apr;628(8006):195-203. Epub 2024 Mar 13 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of Kansas
This is an interesting paper, even though it is not directed toward Alzheimer’s disease. It does make some fundamental observations that are worth noting, and which are pertinent to Alzheimer’s. One is that mitochondrial function influences the activity and the status of immune cells. This is not a new observation, and there are multiple pathways through which mitochondria can influence inflammation and immunologic responses. The reverse electron transport phenomenon the author studied can be added to this canon.
Another is that mitochondria are important for free radical production, which certainly is an observed characteristic of AD. I also note that this study has potential relevance to drug development studies, such as those being pursued by Jania Trushina, in which some are looking at drugs that could potentially affect the reverse electron transport to treat AD.
Make a Comment
To make a comment you must login or register.