Not Just for Proteins—Expanded RNA Repeats Form Gels, Too
Quick Links
In the last two years, researchers have turned up evidence that many proteins involved in neurodegenerative disease form liquid droplets within cells that are analogous to globs of oil in water. Pathogenic mutations harden these natural droplets into semi-solid gels, triggering formation of protein aggregates, according to some research. Now, in the May 31 Nature, researchers led by Ron Vale at the University of California, San Francisco, report that RNA molecules carrying expanded repeats can do something similar. In test tubes and cell cultures, repeat numbers greater than the threshold for causing disease triggered RNA to clump up via base-pairing, forming droplets that often became gel-like. Various chemical agents that disrupt base-pairing broke up the droplets, indicating that this was the mechanism underlying the formation of these RNA foci. “Thinking about RNA not just as a passive substrate, but as an active agent of disease through its ability to form aggregates, might suggest ways of preventing or dissolving foci,” Vale told Alzforum.
Other researchers said the paper makes a valuable contribution. “It’s really exciting to learn that RNA can also form gels. It suggests we should look much harder at the RNA component of granules in the future,” said Simon Alberti at the Max Planck Institute of Molecular Cell Biology and Genetics, Dresden, Germany. Rohit Pappu at Washington University in St. Louis wrote to Alzforum, “For those of us in the Huntington’s disease field, this finding provides a new twist to a complicated and continually evolving story.” (See full comment below.)
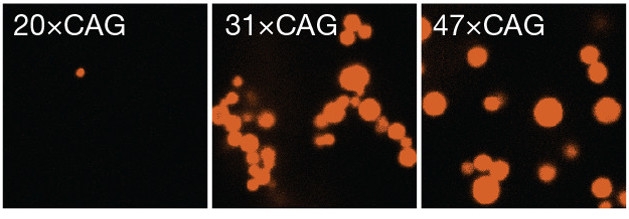
RNA Condensation. In a test tube, CAG repeats above a certain length form gel-like droplets (red). [Courtesy of Jain and Vale, Nature.]
The idea that liquid-liquid phase separation plays a role in neurodegenerative disease took off in 2015, when several groups reported that two proteins involved in amyotrophic lateral sclerosis, FUS and hnRNPA1, formed liquid droplets inside cells (see Oct 2015 webinar). Pathogenic mutations help freeze up these droplets, precipitating aggregates (Sep 2015 news; Oct 2015 news; Oct 2015 news). Later studies added TDP-43, tau, and dipeptide repeat proteins made from the C9ORF72 expansion to the list of proteins that behave this way (Sep 2016 news; Oct 2016 news; May 2017 conference news). All of these proteins contain a disordered or low-complexity sequence that allows them to associate with each other.
The behavior of RNA in these diseases has received less attention. Some studies reported that the nucleic acid might facilitate clustering of dipeptide repeat proteins by neutralizing the proteins’ charge, but did not look at how RNA acted by itself (Oct 2015 conference news; Mar 2017 news). However, repeat expansion diseases such as Huntington’s, spinocerebellar ataxia, and myotonic dystrophy, as well as ALS, share the common feature of nuclear RNA foci, hinting that these structures might be important in disease.
To investigate the factors that give rise to foci, first author Ankur Jain examined how fluorescently labeled RNA of various repeat lengths behaved in a test tube. Jain and Vale found that RNA containing 20 or fewer CAG or CUG repeats remained soluble, while molecules with more than 30 repeats underwent liquid-liquid phase separation, forming tiny droplets. Higher repeat number gave rise to larger droplets (see image above). These RNA droplets behaved like gels rather than liquids. They poorly recovered fluorescence after photobleaching, indicating that individual RNA molecules stayed fixed within the matrix.
Notably, the longer GGGGCC repeat in the C9ORF72 expansion was even stickier, forming liquid droplets at a threshold of five or more repeats in vitro, and generating a mesh-like structure at 10 or more repeats. Hexanucleotide repeats are known to form complex quadruplex structures with each other (Nov 2013 conference news; Reddy et al., 2013; Conlon et al., 2016). The authors hypothesized that this quadruplex structure helped generate the larger, more complex gels seen with the hexanucleotides.
Would the same gelling behavior hold true in cells? The authors turned to the U20S osteosarcoma line often employed to study RNA and protein granules. They transfected the cells with various synthetic CAG repeat lengths, and again found that fewer than about 20 repeats did not trigger foci formation, while longer expansions did. Vale noted that this is about the same threshold repeat length that causes disease in people, with 20-40 repeat carriers exhibiting some symptoms and those with longer expansions having more aggressive disease. In cells, unlike in solution, these CAG droplets behaved like liquids, fusing with each other and recovering quickly after photobleaching. The presence of nuclear proteins such as helicases that remodel base-pairing might keep the foci mobile, the authors speculated. In support of this, depleting cellular ATP, which helicases require, hardened up the droplets. Alberti found this intriguing, noting that mitochondrial activity and ATP production are known to drop with age. He speculated that this mechanism might help explain why the effects of expanded repeat diseases manifest later in life. Researchers have also come to appreciate recently that a major function of RNA-binding proteins may be to prevent the nucleic acid from forming liquid droplets or gels.
As in the cell-free experiments, GGGGCC repeats clumped up more readily than triplet repeats in cells, forming foci at repeat lengths as low as 16. These foci were gel-like, recovering only slowly after photobleaching. Hexanucleotide repeats are known to be more toxic than triplets, Alberti noted. In people, around 23 hexanucleotide repeats are sufficient to trigger disease.
The close correlation between repeat length, gel formation, and disease hints that RNA gels may help poison cells. In an accompanying Nature editorial, Clifford Brangwynne and David Sanders at Princeton University, New Jersey, noted that the formation of liquid droplets at certain repeat lengths “might explain why nucleotide-repeat expansions cause disease only when they reach or exceed a critical length.” However, a direct role in toxicity remains to be shown, researchers agreed. The authors saw no ill effects on cells in their week-old cultures. In addition, Rosa Rademakers and colleagues at the Mayo Clinic in Jacksonville, Florida, reported that the presence of RNA foci did not correlate with neurodegeneration in 63 postmortem brains from people with the C9ORF72 expansion (DeJesus-Hernandez et al., 2017).
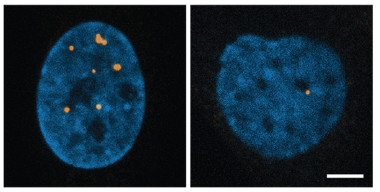
Breaking Up Foci.
RNA foci (orange) dot the nucleus (blue) of fibroblasts from a person with myotonic dystrophy (left), but largely vanish after treatment with a nucleic acid disruptor, the cancer drug doxorubicin (right). [Courtesy of Jain and Vale, Nature.]
If foci are toxic, how might they be treated? Because base-pairing forms RNA foci, agents that disrupt this process should be able to dissolve them, the authors reasoned. Ions with a single positive charge interfere with the pairing of negatively charged amino acids, and so the authors treated cells with ammonium acetate. Within minutes, foci vanished. Likewise, short antisense oligonucleotides lowered the number and size of these RNA clusters. The cancer drug doxorubicin, which worms its way between paired nucleic acids, also busted up foci. To see if the same approach would work in patient cells, the researchers treated fibroblasts from a person with myotonic dystrophy type 1 with doxorubicin, and saw a dramatic drop in the number and size of RNA foci (see image above).
While Vale believes this represents a proof of concept, he noted that doxorubicin also disrupts DNA and is too toxic for therapeutic use in neurodegenerative disease. In ongoing work, he is searching for agents that specifically target only RNA aggregates. Such compounds could be tested in mouse models to see if they reverse disease symptoms, he said. He also wants to examine the cellular effects of RNA foci more closely using inducible expanded-repeat transgenes.
Another unanswered question is how many RNA molecules make up a focus. A previous study reported finding only one RNA occupying each C9ORF72 focus (Feb 2017 news). However, Jain and Vale’s work suggests that multiple RNA transcripts bind to form droplets. Vale noted that they observed multiple RNAs per focus in the fibroblasts from the myotonic dystrophy patient, who carried a CTG expansion.—Madolyn Bowman Rogers
References
Webinar Citations
News Citations
- ALS Protein Said to Liquefy, Then Freeze en Route to Disease
- Do Membraneless Organelles Host Fibril Nucleation?
- FUS Phase Transitions: Liquids and Gels
- Helical Tail Holds Sway Over TDP-43 Packaging
- ALS Research ‘Gels’ as Studies Tie Disparate Genetic Factors Together
- Protein Liquid-Liquid Phase Transitions: The Science Is About to Gel
- Does C9ORF72 Repeat RNA Promote Protein Phase Transitions?
- ALS Dipeptides Drive Liquid-Liquid Phase Separation, Stress Granule Formation
- Researchers Revel in C9ORF72 Advances at RNA Symposium
- At the Heart of Every ALS RNA Aggregate, a Single Transcript
Paper Citations
- Reddy K, Zamiri B, Stanley SY, Macgregor RB, Pearson CE. The disease-associated r(GGGGCC)n repeat from the C9orf72 gene forms tract length-dependent uni- and multimolecular RNA G-quadruplex structures. J Biol Chem. 2013 Apr 5;288(14):9860-6. PubMed.
- Conlon EG, Lu L, Sharma A, Yamazaki T, Tang T, Shneider NA, Manley JL. The C9ORF72 GGGGCC expansion forms RNA G-quadruplex inclusions and sequesters hnRNP H to disrupt splicing in ALS brains. Elife. 2016 Sep 13;5 PubMed.
- DeJesus-Hernandez M, Finch NA, Wang X, Gendron TF, Bieniek KF, Heckman MG, Vasilevich A, Murray ME, Rousseau L, Weesner R, Lucido A, Parsons M, Chew J, Josephs KA, Parisi JE, Knopman DS, Petersen RC, Boeve BF, Graff-Radford NR, de Boer J, Asmann YW, Petrucelli L, Boylan KB, Dickson DW, van Blitterswijk M, Rademakers R. In-depth clinico-pathological examination of RNA foci in a large cohort of C9ORF72 expansion carriers. Acta Neuropathol. 2017 Aug;134(2):255-269. Epub 2017 May 15 PubMed.
Further Reading
Primary Papers
- Jain A, Vale RD. RNA phase transitions in repeat expansion disorders. Nature. 2017 May 31; PubMed.
- Sanders DW, Brangwynne CP. Neurodegenerative disease: RNA repeats put a freeze on cells. Nature. 2017 Jun 8;546(7657):215-216. Epub 2017 May 31 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Washington University in St. Louis
For those of us in the Huntington’s disease field, this finding provides a new twist to a complicated and continually evolving story. The work of Jain and Vale provides a cogent explanation for the long-standing observation of RNA-rich nuclear bodies that are observed in repeat expansion disorders. including HD. What's intriguing is the possibility that these RNA bodies recruit splice factors and enable post-transcriptional processing of repeat expanded RNA. Might transcripts of mutant N-terminal protein fragments of huntingtin be born in these RNA bodies? And if so, as Jain and Vale point out, there might be good reason to target these RNA bodies from a therapeutic standpoint. At a minimum, this is an intriguing observation that provides the HD field with a new hook to approach the problem. It also unifies the RNA and protein worlds through the concept of multivalency.
Of course, the money question is, why are diseases such as HD progressive aging onset disorders? This work doesn't shed light on this issue, but integrating these RNA bodies into a larger systems-centric cellular homeostasis viewpoint might help. It might also help to ask about the neuronal specificity of the formation of these RNA bodies, with a focus on medium spiny versus other neuronal types with regard to HD. Exciting findings open new doors and that's certainly the case with this new work of Jain and Vale.
Make a Comment
To make a comment you must login or register.