Stabilizing C9ORF72 RNA Lowers Toxicity
Quick Links
Hexanucleotide repeat expansions in the C9ORF72 gene cause many cases of amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD), though it is unclear which is most toxic, the expanded RNA or the dipeptide repeats that it encodes. In the November 7 EMBO Molecular Medicine, researchers led by Adrian Isaacs, Pietro Fratta, Rickie Patani, and Stephen Neidle, all at University College London, reported that stabilizing four-stranded G-quadruplex structures assumed by the expanded transcript suppressed accumulation of both RNA and peptides in cortical neurons. The exact mechanism is not yet clear, and since the small-molecule stabilizers do not enter the brain, they do not make for good therapeutic candidates. Nonetheless, the finding serves as a proof-of-concept for this strategy, the authors argued.
- A screen turned up three compounds that stabilized G-quadruplex in expanded C9ORF72 RNA.
- The compounds halved RNA foci and protein deposits in ALS-derived neurons.
- Researchers are searching for brain-penetrant versions to use as therapeutics.
Others agreed. “This is an important study. It opens a new avenue of therapy development that holds a lot of promise,” wrote Jiou Wang at Johns Hopkins University, Baltimore.
Since the discovery of the C9ORF72 expansion in 2011, researchers have debated whether RNA or protein aggregates do most of the dirty work. Recent evidence from animal and cellular models points to dipeptide repeat proteins as the more toxic entity (Aug 2014 news; Dec 2014 news; Sep 2015 news). However, some human studies correlate RNA foci, rather than the dipeptides, with TDP-43 pathology, a hallmark of ALS/FTD (Oct 2013 news; May 2015 news). Some have suggested squelching both RNA and peptides at once by targeting RNA transcription or nuclear export (Aug 2016 news; Jul 2017 news).
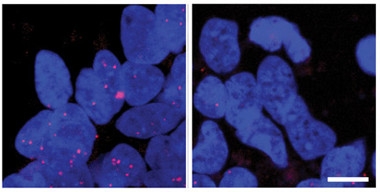
Nuclear cleanup.
Induced cortical neurons from people with expanded C9ORF72 repeats (left) accumulate RNA foci (red) in nuclei (blue), but treatment with an RNA stabilizer (right) slashes deposits. [Courtesy of Simone et al., EMBO Molecular Medicine.]
Isaacs and colleagues took a different approach. They noted that the hexanucleotide repeat naturally formed a stable quadruplex structure through the stacking of its four-repeat guanosine residues (Nov 2013 conference news). Because such G-quadruplexes can regulate RNA processing and translation, the authors wondered what effect stabilizing the structures would have (Bugaut and Balasubramanian, 2012; Conlon et al., 2016). Joint first authors Roberto Simone and Rubika Balendra screened a library of 138 small molecules known to bind G-quadruplexes for those that best maintained the structures as temperatures were increased. They found three structurally related compounds that all bound to and buttressed G-quadruplexes made up of hexanucleotide RNA, but not DNA (see image below).
The authors next tested these molecules on cortical and motor neurons generated from induced pluripotent stem cells derived from patients with C9ORF72 expansions. Four days of treatment with 1μM of these stabilizers cut the number of neurons containing nuclear RNA foci in half, but did not affect accumulation of dipeptide repeat proteins. To curtail protein deposits, the authors had to up the dose to 8–16 μM and treat for seven days. At the highest dose, the most effective of the three stabilizers, dubbed DB1273, lowered levels of polyglycine/proline dipeptides by more than half. The treatment neither compromised cell survival nor affected levels of an unrelated G-quadruplex RNA, suggesting specificity for C9ORF72.
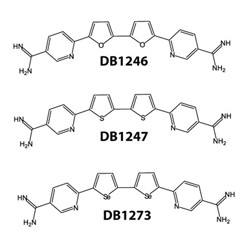
RNA binders.
Three structurally related molecules bound to C9ORF72 RNA and stabilized its G-quadruplexes. [Courtesy of Simone et al., EMBO Molecular Medicine.]
To see if the strategy would work in vivo, the authors fed DB1273 to fruit fly larvae that harbored an expanded C9ORF72 repeat. Deposits of polyglycine/arginine protein dropped by a third. In addition, slightly more larvae survived to the pupal stage, suggesting reduced toxicity. This occurred despite very little DB1273 entering the central nervous system, the authors noted. In ongoing work, the researchers are looking for stabilizers with better brain penetration, Isaacs told Alzforum.
How the stabilizers quell toxicity is not yet known, but Isaacs noted that G-quadruplexes in promoter regions can block translation of RNA (Wolfe et al., 2014). Thus, locking RNA into this structure might directly prevent production of dipeptide repeat proteins. Another study found that potential quadruplex regions tend to stay unfolded in eukaryotic cells, probably due to the presence of helicases and other enzymes that detangle them (Guo and Bartel, 2016). As for reduction of RNA foci, the researchers speculated that physically stabilizing the quadruplex might promote degradation of the expanded RNA by RNases.—Madolyn Bowman Rogers
References
News Citations
- C9ORF72 Killer Dipeptides Clog the Nucleolus
- Live-Cell Studies Blame Arginine Peptides for C9ORF72’s Crimes
- C9ORF72 RNA Foci Acquitted of Toxic Charge—in Fruit Flies
- RNA Deposits Confer Toxicity in C9ORF72 ALS
- Antisense RNA from C9ORF72 Repeats Is Likely Culprit in Patient Neurons
- Tackling Mutant C9ORF72 Transcripts at the Source
- Barring Export of Expanded Repeat RNAs Tames Their Toxicity
- Researchers Revel in C9ORF72 Advances at RNA Symposium
Paper Citations
- Bugaut A, Balasubramanian S. 5'-UTR RNA G-quadruplexes: translation regulation and targeting. Nucleic Acids Res. 2012 Jun;40(11):4727-41. Epub 2012 Feb 20 PubMed.
- Conlon EG, Lu L, Sharma A, Yamazaki T, Tang T, Shneider NA, Manley JL. The C9ORF72 GGGGCC expansion forms RNA G-quadruplex inclusions and sequesters hnRNP H to disrupt splicing in ALS brains. Elife. 2016 Sep 13;5 PubMed.
- Wolfe AL, Singh K, Zhong Y, Drewe P, Rajasekhar VK, Sanghvi VR, Mavrakis KJ, Jiang M, Roderick JE, Van der Meulen J, Schatz JH, Rodrigo CM, Zhao C, Rondou P, de Stanchina E, Teruya-Feldstein J, Kelliher MA, Speleman F, Porco JA Jr, Pelletier J, Rätsch G, Wendel HG. RNA G-quadruplexes cause eIF4A-dependent oncogene translation in cancer. Nature. 2014 Sep 4;513(7516):65-70. Epub 2014 Jul 27 PubMed.
- Guo JU, Bartel DP. RNA G-quadruplexes are globally unfolded in eukaryotic cells and depleted in bacteria. Science. 2016 Sep 23;353(6306) PubMed.
Further Reading
Primary Papers
- Simone R, Balendra R, Moens TG, Preza E, Wilson KM, Heslegrave A, Woodling NS, Niccoli T, Gilbert-Jaramillo J, Abdelkarim S, Clayton EL, Clarke M, Konrad MT, Nicoll AJ, Mitchell JS, Calvo A, Chio A, Houlden H, Polke JM, Ismail MA, Stephens CE, Vo T, Farahat AA, Wilson WD, Boykin DW, Zetterberg H, Partridge L, Wray S, Parkinson G, Neidle S, Patani R, Fratta P, Isaacs AM. G-quadruplex-binding small molecules ameliorate C9orf72 FTD/ALS pathology in vitro and in vivo. EMBO Mol Med. 2018 Jan;10(1):22-31. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.